Terms and Conditions:
BY USING THE SITE OR BY CLICKING ANY "I ACCEPT" BELOW, YOU SIGNIFY YOUR AGREEMENT TO THESE TERMS AND CONDITIONS. IF YOU DO NOT AGREE TO THESE TERMS AND CONDITIONS, DO NOT USE THE SITE.
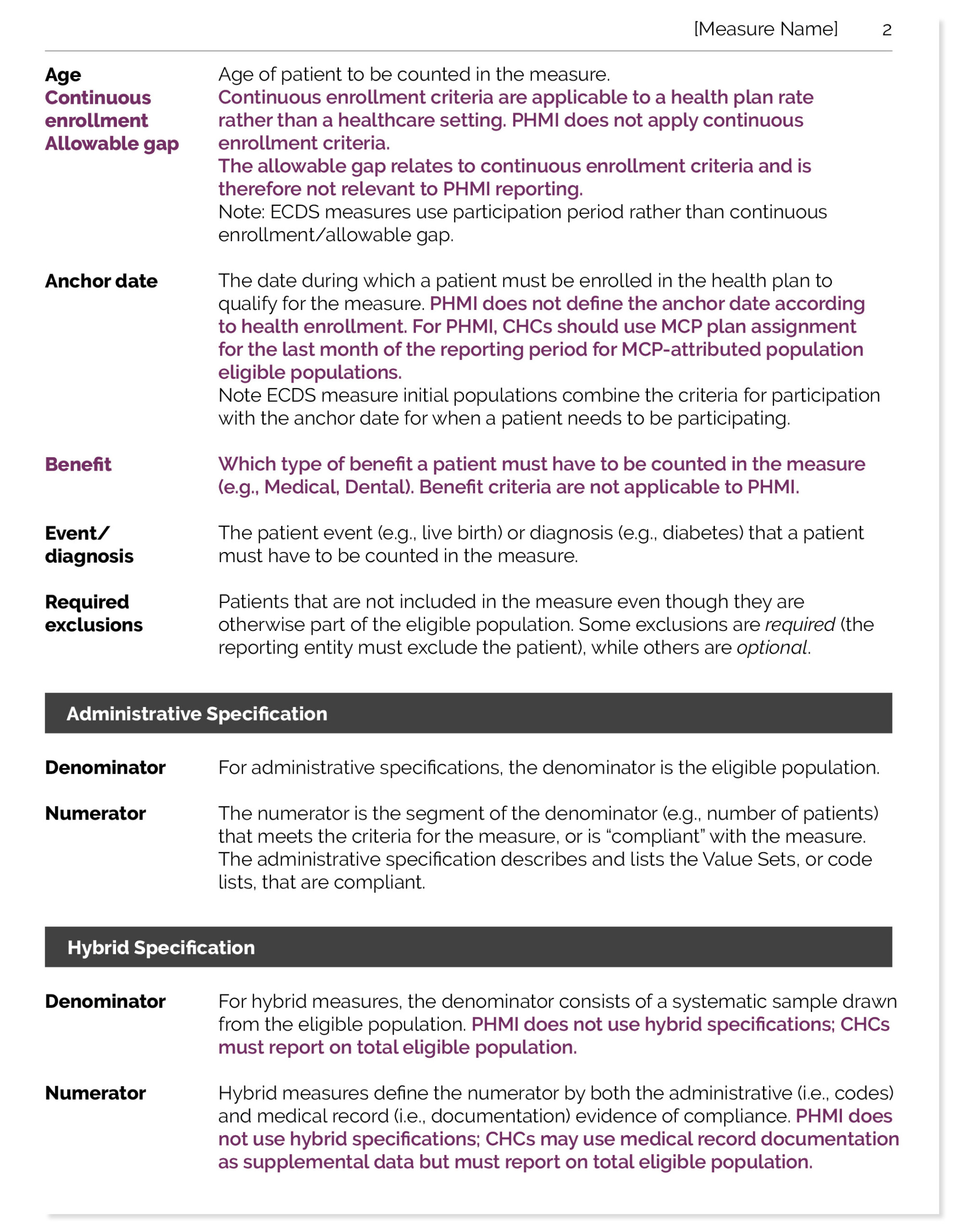
Purpose
Kaiser Foundation Hospitals (“Kaiser”) provides a website as part of its population health management initiative at "https://phminitiative.com/" (the “Site”).
The Site is provided by Kaiser only for use (i) with the California Department of Health Care Services’ initiatives, and (ii) by certain community health centers participating in Kaiser’s population health management initiative (“PHMI”), all as such are located in California (“Permitted Users”) and only for such users respective internal business purposes in relation to the foregoing initiatives. You represent and warrant that you are a Permitted User and using the Site for such internal business purpose. If you are not a Permitted User, for example you are using the Site for a different purpose (e.g., use outside of PHMI) then Kaiser shall have no liability (please Disclaimer and Limitation of Liability section below) or obligation to you, your use of the Site, or the results of use of the Site or the materials, documents, or information contained therein. Additionally, the Site and materials, documents, and information contained therein are provided “as is” and “as available” without any representations or warranties of any sort (express or implied) and you are solely responsible for all liabilities arising from the use of such, including the results arising from such.
If you are a California community health center with a PHM Program Agreement with Kaiser, then the PHM Program Agreement remains in full force and effect and if there is a conflict, additional terms, or inconsistency between these Terms and Conditions and such PHM Program Agreement, then the PHM Program Agreement shall control and govern.
Please note that Kaiser reserves the right to discontinue the Site, and if discontinued, the Site will no longer be available.
The Site allows users to:
- view health-related information
- obtain guides and other materials and tools
- obtain reports with regard to the information the user enters into the Site
The information provided on the Site is not a substitute for independent review and analysis of information entered by the user into the Site and is not clinical or medical advice or recommendations, or the advice of a personal physician or other qualified health care professional and does not constitute a diagnosis or any professional treatment recommendation and you remain solely responsible and liable for such. Never disregard professional recommendations, medical advice or delay in seeking it because of something you have read on the Site. You understand and agrees that Kaiser, through the Site, is not engaged in the practice of medicine and that the Site and the data analytics or results generated therefrom, are information tools and services only, and are not a substitute for competent medical advisors, judgement, and decision making. Only authorized users who are competent healthcare professionals may use the Site and users thereto will rely on their own clinical discretion, decision making, and judgement in any diagnosis and treatment. All medical practice management and patient care decisions made in or from the outputs or results of such Site are exclusively the user’s responsibility. You agree that Kaiser has no liability whatsoever for any and all claims that any improper medical treatment or decision resulted from or arose out of use or reliance upon the Site or outputs thereof. You are solely responsible for complying with all laws and regulations relative to your use of the Site. You agree that with respect to the Site, Kaiser makes no representation or recommendation whatsoever as to the appropriate plan of care, selection of treatment for any individual or groups, or the presence or non-presence of a given condition or corresponding diagnosis or billing code and determination of such remains your sole and exclusive obligation and responsibility.
The Site only presents reporting for the users’ review and consideration, and the user is solely and exclusively responsible and liable for use of the Site, and for independently validating the information entered by the user into the Site, and the use of the information and reporting provided by the Site.
Information Uploaded or Transmitted to the Site
You are solely responsible for the information you upload or transmit to the Site. You agree that you will not upload or transmit any information or content of any type that infringe upon, misappropriate or violate any rights of any party. You represent and warrant that you have sufficient rights and permissions to provide such information and data.
Personal Information
Any personal information you submit to the Site is governed by our Site and Privacy Statement and you agree to such. (A link to our Site Privacy Statement is available at the bottom of each page on our Site) This includes information on your rights to see and receive copies of your own or others' personal health information. The Site does not need any protected health information (“PHI”), as defined by the Health Insurance Portability and Accountability Act (“HIPAA”), and you represent and warrant that you will not provide any PHI to the Site. If you do provide PHI in contravention of the above, Kaiser will not be responsible or liable for such PHI.
Passwords
If Kaiser provides you with a password, you are responsible for taking all reasonable steps to ensure that no unauthorized person shall have access to your Kaiser online password or account. If Kaiser provides you with a password, it is your sole responsibility to (1) control the disclosure and use of your activation codes, password reset codes and password; (2) authorize, monitor, and control access to and use of your Kaiser online account and password; and (3) promptly change your password if you feel it has become compromised; and (4) promptly inform the Site manager of any need to deactivate an account entirely.
Breaches of These Terms and Conditions
In consideration of being allowed to use the Site interactive services, you agree that the following actions shall constitute a material breach of these Terms and Conditions:
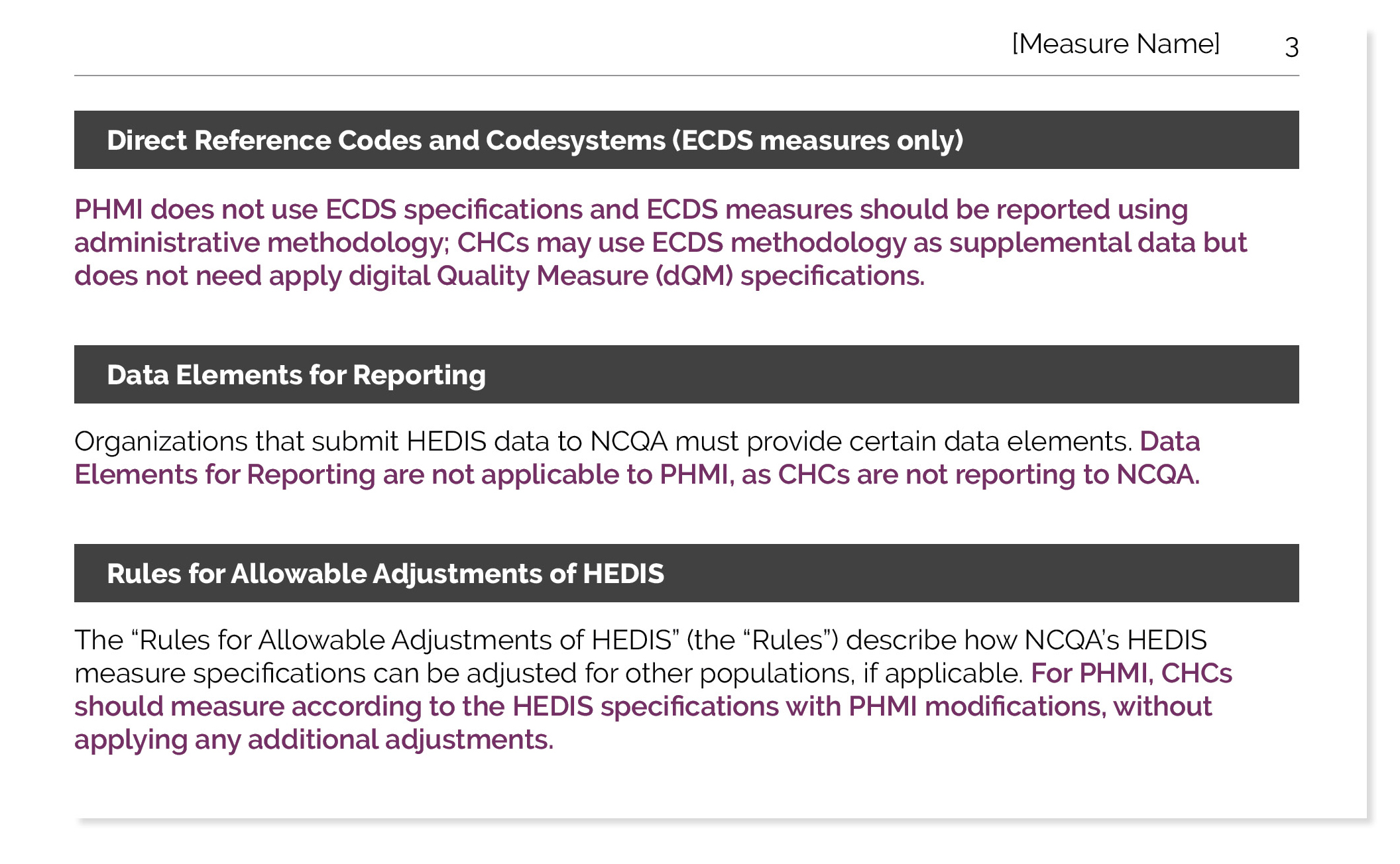
- signing on as or pretending to be another company, entity or person
- transmitting material that infringes or violates the intellectual property rights of others or the privacy or publicity rights of others or transmitting information or materials that comprises PHI
- transmitting material that is unlawful, obscene, defamatory, predatory of minors, threatening, harassing, abusive, slanderous, or hateful to any person (including Kaiser personnel) or entity as determined by Kaiser in its sole discretion
- using interactive services in a way that is intended to harm, or a reasonable person would understand would likely result in harm, to the user or others
- collecting information about others, including email addresses
- intentionally distributing viruses or other harmful computer code
- attempting to (1) probe, scan, "hack", or test the vulnerability of the Site or any Kaiser system or network; or (2) breach any security or authentication measures on the Site or any Kaiser system connected to either the Site
- using any "deep-link", "page-scrape", "robot", "spider", data mining tools, data gathering and extraction tools, or other automatic device, program, algorithm or methodology, to (1) access, acquire, copy or monitor any portion of the Site, or (2) in any way reproduce or circumvent the navigational structure or presentation of the Site
- altering, modifying or otherwise changing any content, including any materials or downloads, on or available through the Site or using such for any purpose not authorized under these Terms and Conditions
Kaiser expressly reserves the right, in its sole discretion, to terminate a user's access to any interactive services and/or to any or all other areas of the Site due to any act that would constitute a violation of these Terms and Conditions.
Kaiser may offer interactive areas of the Site under license with Third Party software and service providers. These Terms and Conditions extend to and apply to your use of our licensors’ software. Our licensors are direct third-party beneficiaries of applicable rights under these Terms and Conditions. By using our licensor’s software, you agree to be bound directly to our licensors with respect to your compliance with these Terms and Conditions. Kaiser may directly enforce the licensors’ rights under these Terms and Conditions on the licensors’ behalf or a licensor may enforce its rights under these Terms and Conditions directly against you or other users who breach these Terms and Conditions.
Communications By Email
By accepting these Terms and Conditions, you agree to receive essential communications by email. These communications may include (but are not limited to):
- notification that an important message awaits you on the Site
- Site and/or service updates
- emergency alerts and critical messages (for example, Site being down)
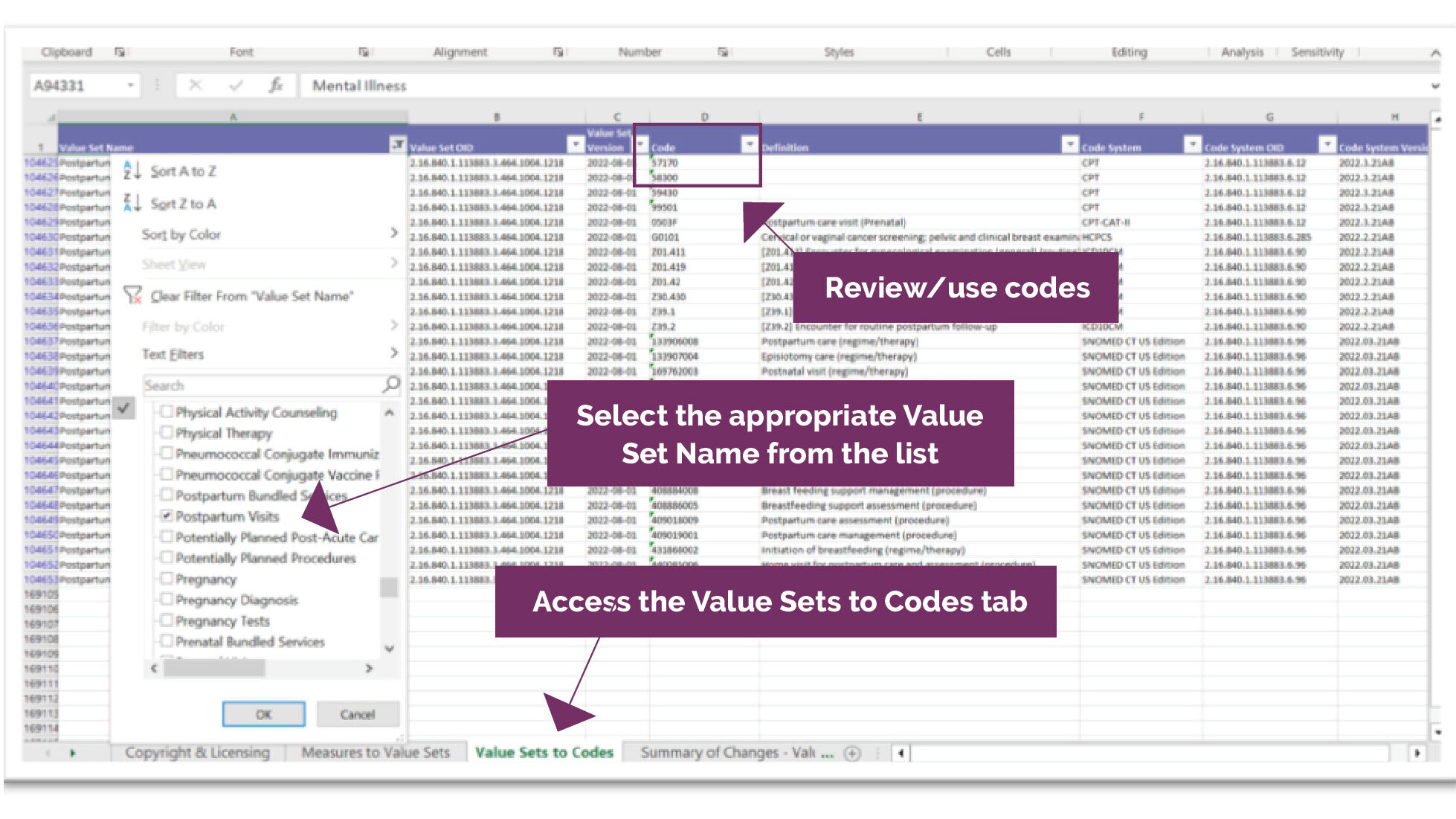
You should take appropriate precautions to protect personal and confidential information, and to use your computers and devices in a secure and responsible manner. We are not responsible for the security of your computers and devices and we expect that you will configure them in a secure and responsible manner.
We may use a secure transmission method to send you communications through e-mail and/or our Site. While such transmission methods provide reasonable protections against unauthorized access, no system can perfectly guard against risks of intentional intrusion or inadvertent disclosure of information sent to us. Moreover, when you transmit information via the internet, your information will be transmitted over a medium that is beyond our control, and therefore the security of the transmission may be compromised before it reaches us. Accordingly, we make no guarantee as to confidentiality or security. If you have concerns regarding the electronic transmission of information, you should consider using non-electronic communication methods to receive documents relating to health plan coverage.
All Kaiser staff and vendors who have access to, or are involved with, the processing of personal information are trained to respect the confidentiality of your personal information.
Use By Children
We do not knowingly allow minors under the age of 13 to create accounts that allow access to the secured features of the Site.
Access, correction, and data integrity
Although we attempt to maintain the integrity and accuracy of the information on the Site, we make no guarantees as to its correctness, completeness, or accuracy. The Site may contain typographical errors, inaccuracies, or other errors or omissions. Also, unauthorized additions, deletions, or alterations could be made to the Site by third parties without our knowledge. If you believe that information found on the Site is inaccurate or unauthorized, please inform us.
Revisions, Changes, And Updates
We may revise the information on the Site or otherwise change or update the Site, including these Terms and Conditions, without notice to you. Kaiser may also make improvements and/or changes in products and/or services described on the Site or add new features at any time without notice. We encourage you to periodically read these Terms and Conditions to see if there have been any changes to our policies that may affect you. Your continued use of the Site will signify your continued agreement to these Terms and Conditions as they may be revised.
Links to Third Party Websites
The Site provides links to other websites that are not owned or controlled by Kaiser ("Third Party Websites"). We provide links to Third Party Websites as follows.
- Kaiser provides links to Third Party Websites to connect you easily to additional sources of health information or third party services that may be of interest to you. We may not have any business relationship with the party that controls this type of Third Party Website and a link to such a site is offered only as a convenience to you.
- Kaiser also provides links to Third Party Websites managed by vendors that we have made arrangements with to offer you services to help you manage your health or to take and fulfill orders when you purchase items or materials from us. These Third Party Websites may be co-branded, meaning that they display the Kaiser Permanente logo and the logo of the Third Party vendor but they are owned and controlled by the Third Party.
In each such instance, where practicable, we will let you know when you are leaving the Site and linking to a Third Party Website. We may use an icon that we identify with an appropriate legend to let you know when you are leaving the Website.
Kaiser may not be responsible for the content, security or the privacy practices of Third Party Websites. Please review the privacy statement and any terms of use of each Third Party Website you visit, if applicable. Unless we specifically advise you otherwise, links to Third Party Websites do not constitute or imply endorsement by Kaiser of those sites, the information they contain or any products or services they describe. Kaiser Permanente does not receive payment or other remuneration in exchange for you linking to or using any Third Party Website.
Hardware and Software Requirements
In order to access and retain your electronic documents, you will need the following computer hardware and software:
- A computer with an internet connection
- A current web browser that includes 128-bit encryption (e.g., Microsoft Edge , Firefox version 71 or later, Chrome version 49 or later, or Safari version 11 or later) with cookies enabled
- Current Adobe Acrobat Reader version to open documents in PDF format
- A valid email address (your primary email address in your Site profile)
- Sufficient storage space to save past documents or an installed printer to print them
By giving your consent at this time, you agree that you have access to the necessary hardware and software as listed above, and are able to receive, open, and print or download a copy of any document for your records.
We will notify you if there are any material changes to the hardware or software needed to receive electronic documents from Kaiser at least 30 days in advance of the date any such changes are made. At that time, we will also provide instructions on how to withdraw, change, or renew your consent for electronic delivery of your documents.
Updating Your Contact Information
It is your responsibility to keep your primary email address up to date so that Kaiser can communicate with you electronically.
You understand and agree that if Kaiser sends you an email notification that a communication and/or document was sent and/or an electronic document has been posted, and we do not receive a notification of delivery failure (i.e., a “bounce message”), Kaiser will be deemed to have provided the communication to and/or document you. This could occur if, for example, you do not receive it because your primary email address on file is blocked by your service provider, or you are otherwise unable to receive electronic communications.
Please note that if you use a spam filter that blocks or re-routes emails from senders not listed in your email address book, you must add the Kaiser email address to your email address book so that you will be able to receive the communications we send to you (please consult the instructions of your spam filter for specific directions on how to do this).
DISCLAIMER AND LIMITATION OF LIABILITY
Disclaimer of Warranties
THE SITE, AND ANY CONTENT, INFORMATION, SERVICES OR PRODUCTS OBTAINED THROUGH THE SITE IS PROVIDED "AS IS," WITH ALL FAULTS, WITH NO REPRESENTATIONS OR WARRANTIES OF ANY KIND, EITHER EXPRESSED OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, THE IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR NON INFRINGEMENT. YOUR USE OF THE SITE IS VOLUNTARY, AND AT YOUR OWN RISK. ANY REFERENCES TO SPECIFIC PRODUCTS OR SERVICES ON THE SITE DOES NOT CONSTITUTE OR IMPLY A RECOMMENDATION OR ENDORSEMENT OF SUCH PRODUCTS OR SERVICES BY KAISER UNLESS SPECIFICALLY STATED OTHERWISE.
Limitation of Liability
KAISER AND ITS PARENT, AFFILIATES, RELATED ENTITIES, SUPPLIERS, LICENSORS AND OTHER THIRD PARTIES MENTIONED OR LINKED TO ON THE SITE ARE NEITHER RESPONSIBLE NOR LIABLE FOR ANY DIRECT, INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, EXEMPLARY, PUNITIVE, OR OTHER DAMAGES (INCLUDING, WITHOUT LIMITATION, THOSE RESULTING FROM LOST PROFITS, LOST DATA, OR BUSINESS INTERRUPTION) ARISING OUT OF OR RELATING IN ANY WAY TO THE SITE, AND ANY CONTENT, INFORMATION, SERVICES OR PRODUCTS OBTAINED THROUGH THE SITE, ANY APP TO WHICH YOU PERMIT KAISER TO RELEASE DATA (AND INCLUDING, WITHOUT LIMITATION, THE APP'S USE OF ANY DATA YOU MAKE AVAILABLE TO THE APP), AND/OR ANY THIRD PARTY WEBSITE, OR YOUR USE OF ANY OF THE FOREGOING, WHETHER BASED ON WARRANTY, CONTRACT, TORT, OR ANY OTHER LEGAL THEORY AND WHETHER OR NOT ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. EXCEPT AS DESCRIBED IN THE FOLLOWING PARAGRAPH, YOUR SOLE REMEDY FOR DISSATISFACTION WITH THE SITE, AND/OR THIRD PARTY WEBSITES IS TO STOP USING THE SITE.
APPLICABLE LAWS MAY NOT ALLOW SUCH DISCLAIMER OF WARRANTIES, LIMITATIONS OF LIABILITY, OR THE EXCLUSIONS FROM SUCH LIABILITY, AND YOU MAY BE ENTITLED TO SEEK OTHER REMEDIES. IN SUCH CASE, THE ABOVE DISCLAIMERS, LIMITATIONS OR EXCLUSIONS MAY NOT APPLY TO YOU.
Indemnification
You agree to defend, indemnify and hold Kaiser, the other Kaiser Permanente entities, Kaiser’s licensors and service providers, and their respective officers, directors, employees and agents (each an “Indemnified Party”) harmless from and against all liabilities, damages, claims, costs and expenses (including reasonable attorneys’ fees and costs and expenses of expert witnesses) or other losses (collectively, “Losses”) brought by any third party, including without limitation patients or governmental or regulatory entities, against an Indemnified Party arising out of or otherwise in relation to, directly or indirectly, your, or your end users’, employees’, affiliates’, medical providers’, contractors’, subcontractors’ or agents’, acts or omissions.
Choice of Law
THESE TERMS AND CONDITIONS ARE GOVERNED BY CALIFORNIA LAW WITHOUT REGARD TO ITS PRINCIPLES OF CONFLICTS OF LAW. IF ANY VERSION OF THE UNIFORM COMPUTER INFORMATION TRANSACTIONS ACT (UCITA) IS ENACTED AS PART OF THE LAW OF CALIFORNIA, THAT STATUTE SHALL NOT GOVERN ANY ASPECT OF THESE TERMS AND CONDITIONS.
Copyrights
Except as otherwise indicated, the Site and all content on the Site, including text, graphics, logos, button icons, photos, images, forms, audio, video, questionnaires, information processing methodology and/or approaches, and software, is the property of Kaiser, its related entities or its licensors and is protected by United States and international copyright laws. Kaiser allows you to view or download a single copy of the material on the Site solely for your personal, noncommercial use.
The compilation of all content on the Site is the exclusive property of Kaiser or, as applicable, its licensors, and is protected by United States and international copyright laws. Unless specifically authorized in writing by Kaiser, any use of these materials, or of any materials contributed to the Site by entities other than Kaiser, on any other website or networked computer environment for any purpose is prohibited.
Any rights not expressly granted by these Terms and Conditions or any applicable end-user license agreements are reserved by Kaiser. Content and features are subject to change or termination without notice in the editorial discretion of Kaiser.
The Digital Millennium Copyright Act of 1998 (the "DMCA") provides recourse for copyright owners who believe that material appearing on the Internet infringes their rights under U.S. copyright law. If you believe in good faith that materials appearing on the Site infringe your copyright, you (or your agent) may send us a notice requesting that the material be removed, or access to it blocked.
In addition, if you believe in good faith that a notice of copyright infringement has been wrongly filed against you, the DMCA permits you to send us a counter-notice. Notices and counter-notices must meet statutory requirements imposed by the DMCA. One place to find more information is the U.S. Copyright Office website, currently located at https://www.copyright.gov.
In accordance with the DMCA, Kaiser has designated an agent to receive notification of alleged copyright infringement in accordance with the DMCA. Any written Notification of Claimed Infringement should comply with Title 17, United States Code, Section 512(c)(3)(A) and should be provided in writing to our designated agent as follows:
Agent: kp.org Copyright Compliance Department
Address: Kaiser Permanente, KP Digital, 4460 Hacienda Drive, Building A, 3rd floor, Pleasanton, CA 94588
Fax: 1-925-737-2276
Phone: 1-925-598-2799
Email: ISG.QA-Compliance@kp.org
(This telephone number is for copyright-related complaints only. No solicitations.)
Please note: If you materially misrepresent that online material, product, or activity is infringing your copyrights, you may be liable for damages (including court costs and attorneys' fees) and could be subject to criminal prosecution for perjury. We suggest that you consult your legal advisor before filing a notice or counter-notice.
Trademarks And Service Marks
"Kaiser Permanente" and the Kaiser Permanente logo are registered service marks of Kaiser Foundation Health Plan, Inc. Other proprietary marks of Kaiser or third parties may be designated as such from time to time on the Site through use of the TM, SM, or ® symbols. Users of the Site are not authorized to make any use of the Kaiser marks (including without limitation PHMI and/or PHMI Program branding) or the proprietary marks of third parties, including but not limited to, as metatags or in any other fashion that may create a false or misleading impression of affiliation or sponsorship with or by Kaiser Permanente, its related entities, or the applicable third party.
Copyright © 2024 Kaiser Foundation Health Plan. All rights reserved for all countries.
Last updated: June 2024
Version 1