©️ 2024 Kaiser Foundation Health Plan, Inc.
This guide provides step-by-step guidance for improving population-based care for adults with preventive care needs with the goal of supporting substantive cultural, technological and process changes. In particular, it focuses on increasing screening rates for colorectal cancer, breast cancer and cervical cancer.
This guide was designed as part of the Population Health Management Initiative (PHMI), a California collaboration of the Department of Health Care Services (DHCS), Kaiser Permanente and Community Health Centers. Much of the content is relevant and adaptable to primary care practices of all kinds working to improve the health of the populations they serve.
According to the U.S. Centers for Disease Control and Prevention (CDC), in 2020[1] colorectal cancer, female breast cancer and cervical cancer resulted in over 98,000 deaths in the U.S. and over 10,000 deaths in California.
|
Cancer Type |
Location |
New Cases 2020 |
Deaths 2020 |
|---|---|---|---|
Colorectal cancer |
United States |
126,240 |
51,869 |
California |
13,447 |
5,401 |
|
Female breast cancer |
United States |
239,612 |
42,273 |
California |
25,809 |
4,520 |
|
Adults living with chronic conditions |
United States |
11,542 |
4,272 |
California |
1,346 |
489 |
One of our more powerful tools in the fight against these three cancers is having all patients complete all the U.S. Preventive Services Task Force’s (USPSTF) recommended screenings. Despite the known benefits of screening, screening rates for these three cancers are suboptimal.[2]
|
Cancer Screening Type |
California Screening Rate |
National Rank |
U.S. Screening Rate |
|---|---|---|---|
Up-to-date stool test, endoscopy and colonoscopy; 45 to 75 years; 2020 |
53% |
52 |
64% |
Up-to-date mammography; people 40 to 74 years; 2020* |
60% |
49 |
67% |
Up-to-date Pap smear and human papillomavirus (HPV) test; people 21 to 65 years; 2020* |
87% |
25 |
87% |
*The USPSTF is currently in the process of updating these guidelines, and clinical teams should review progress in order to incorporate current guidance.
Developing, implementing and continually improving a multifaceted and culturally relevant cancer screening protocol that includes all patients is critically important for a range of reasons:
- It helps avoid missed or delayed diagnosis, which is devastating to patients and their family and caregivers.
- A culturally relevant screening program can help to address inequities in access and outcomes by tailoring outreach and education to the populations served by your practice.
- It helps practices adhere to the most current cancer screening guidelines.
The work to ensure that all adults receive all recommended cancer screenings is a continuous effort and we still have much to learn. This living document uses existing evidence, bright spots and examples from the field to offer practical guidance on improving the effectiveness of your cancer screening protocols. While some of the guidance in this document is technical, much of the guide is focused on supporting practices in the substantive cultural, technological and process changes that lead to improved population-based care for adults. Virtually every activity in the guide will require some level of adaptation for your practice’s unique context. Population Health Management Initiative (PHMI) will update this guide as we learn from and with practices.
We have organized the key activities in this guide into three categories:
- Foundational activities: Activities that all practices should implement as part of their cancer screening protocol.
- Going deeper activities: More advanced activities that build off the key activities and that help to ensure your practice can achieve equitable improvement in your cancer screening rates.
- On the horizon activities: Additional activities, including ideas worthy of testing that include the latest ideas and thinking on cancer screening.
Sequencing activities: We recommend that practices consider planning and attempting to implement the activities in the sequence provided in this guide. At the same time, we recognize that different practices may follow a different path toward prioritizing and implementing these activities. Furthermore, there is overlap between activities; many activities build off or from the building blocks of other activities.
Testing and implementing: For each activity, we provide guidance on how to plan, test and implement the activity along with links to other resources, technology considerations and examples. Consider testing different versions of the action steps and roles on a small scale before fully implementing at your practice.
Maintaining the progress: For many activities we have also provided tips for periodically reviewing and making improvements to key workflows even after initially implementing the change. Ongoing review and continual improvement are important for your practice to maintain your progress in population health management and help you stay nimble in adapting to changing patient demographics, new clinical best practices, new payment policies, workforce changes and other changes at your practice.
If your practice implements the foundational activities in this guide, you should be able to achieve the following key competencies.For adult preventive care your practice will be able to consistently:
- Engage patients served by your practice to validate any of your proposed process improvements and to propose alternative methods to improve quality in your focus area.
- Analyze core quality measures to identify inequities and improvement opportunities for colorectal, breast and cervical screening rates.
- Use evidence-based clinical guidelines, identify when and where it is necessary to update, or develop new protocol(s) for colorectal, breast and cervical screening.
- Create an outreach protocol to reach and engage all attributed patients.
- Create a health-related social needs screening process that informs patients’ treatment plans.
- Assess current health information technology (HIT) capabilities and develop a plan for ongoing improvement in data utilization, care team workflows, and efficiency.
This guide also includes sections on measurement, equity, social health and behavioral health integration and an appendix including helpful tools and resources. We have included information about California Medi-Cal-covered benefits and services that were up to date at the time of publishing, but benefits and billing guidance change over time. Nothing in this guide should be considered formal guidance, and anyone using this guide should check with the appropriate authorities on benefits and billing guidance. This document will be refined based on continued learning on this topic and may include additional activities, examples, resources and sections in the future.
Improving the health of a population impacts everyone in a practice. Critical roles needed to engage in the work outlined in this guide and support practice change include:
- Quality improvement leadership, like a director of quality improvement (QI), to support cultural changes.
- Coaches or practice facilitators who are partnered with teams to help identify areas for improvement and support change through change management strategies.
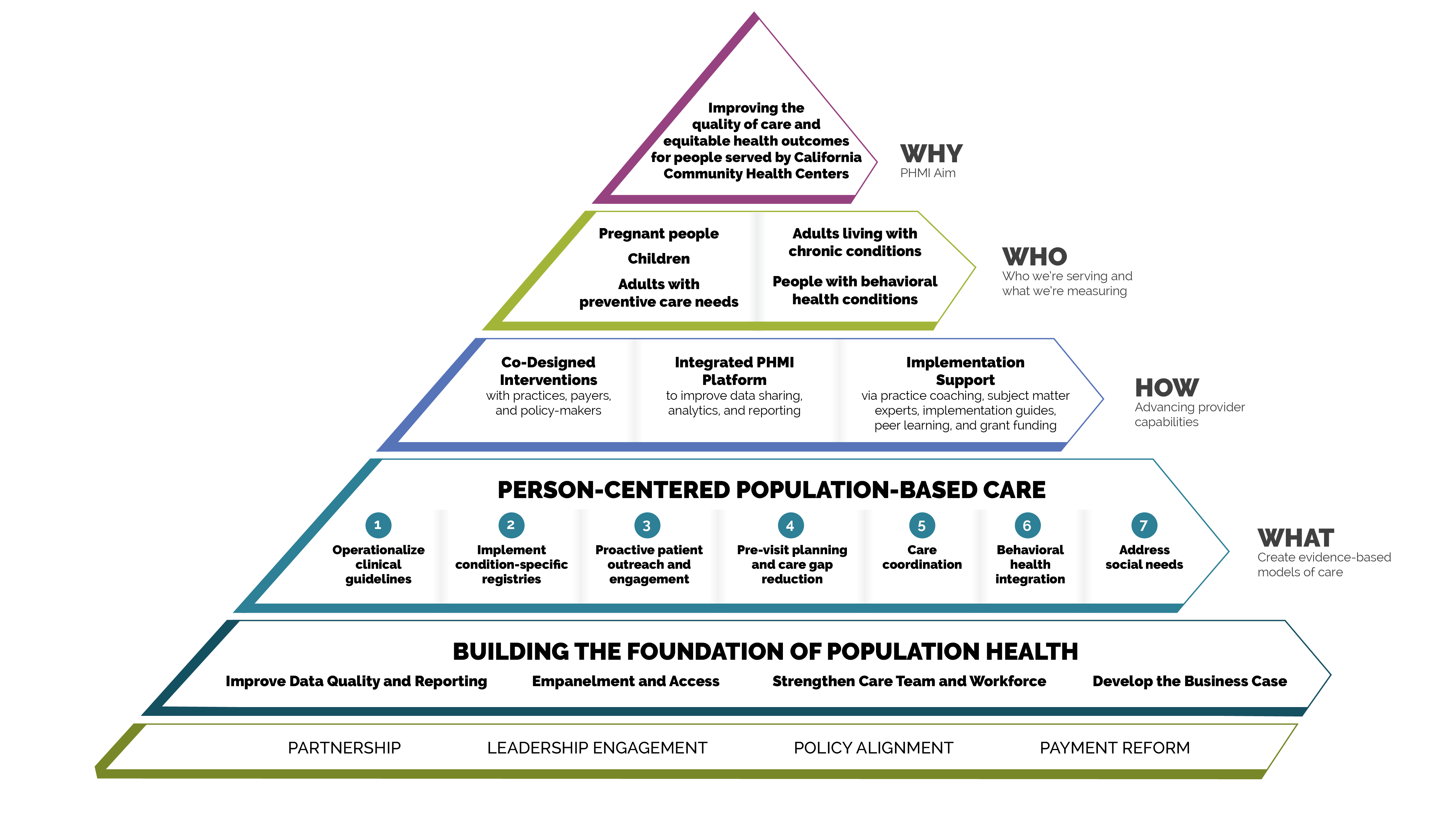
Putting the Key Activities in Context
Person-centered population-based care
Each of the key activities advance one or more of the seven person-centered population-based care change concepts:
- Operationalize clinical guidelines.
- Implement condition-specific registries.
- Proactive patient outreach and engagement.
- Pre-visit planning and care gap reduction.
- Care coordination.
- Behavioral health integration.
- Address social needs.
FIGURE 1: PHMI IMPLEMENTATION MODEL
The measures covered in this guide consist of Healthcare Effective Data and Information Set (HEDIS) measures designated as core and supplemental measures by PHMI. These measures can be considered outcome measures because there is ample evidence that improved timely care will improve overall population health outcomes for adults requiring cancer screening. All measures use standard HEDIS definitions and are aligned with California Advancing and Innovating Medi-Cal (CalAIM) and Alternative Payment Methodology (APM) 2.0. For information about these measures, reference the PHMI Data Quality and Reporting Guide.
PHMI has selected one core and two supplemental measures of focus for adults with preventive care needs. Practices can track other measures that feel important and relevant. This guide provides detailed guidance to improve your practice’s results on the following four core and supplemental measures:
- Colorectal Cancer Screening (Core Measure).
- Breast Cancer Screening (Supplemental Measure).
- Cervical Cancer Screening (Supplemental Measure).
Core HEDIS measures for PHMI
|
PHMI Populations of Focus |
Measures |
|---|---|
Adults with preventive care needs |
Colorectal Cancer Screening |
Supplemental HEDIS measures for PHMI
|
PHMI Populations of Focus |
Measures |
|---|---|
Adults with preventive care needs |
Breast Cancer Screening |
|
Cervical Cancer Screening
|
The core and supplemental measures are part of a larger measurement strategy and learning system, as outlined in Appendix A: Sample, Idealized System Diagram: Weaving Your Measurement Strategy and Learning System into Practice Operations. Key Activity 1: Convening a Multidisciplinary Implementation Team for Cancer Screening outlines how your practice can develop a robust measurement system to support this work. In addition to quality assurance and monitoring, measures are also used during practice operations alongside other data for learning to:
- Guide the actions of the multidisciplinary implementation team as they use a systematic approach to decreasing inequities and implementing key activities across the practice.
- Support the care team’s efforts to advance population health and reduce care gaps through daily, weekly and monthly workflows, as well as continuous identification of opportunities for improvement.
The PHMI Clinical Guidelines Advisory Group (CGAG) was established to create a standardized approach to review, adopt and promote established clinical guidelines in the PHMI cohort. For more information, please see the PHMI Clinical Practice Guidelines for Key Medi-Cal Populations of Focus.
Colorectal cancer screening clinical guidelines
FIGURE 2: CLINICAL GUIDELINES: COLORECTAL CANCER SCREENING
Guideline source |
|
PHMI measure |
Colorectal Cancer Screening |
Guideline language |
Conduct colorectal cancer screening for persons aged 45 to 75 using any of the following screening modalities and intervals:
|
Breast cancer screening clinical guidelines
FIGURE 3: CLINICAL GUIDELINES: BREAST CANCER SCREENING
Guideline Source |
|
PHMI Measure |
Breast Cancer Screening |
Guideline Language |
Age to start Mammography (USPSTF): Biennial screening mammography is recommended for women aged 40 y/o. The decision to start screening mammography in women prior to age 40 y/o should be an individual one. |
Cervical cancer screening clinical guidelines
FIGURE 4: CLINICAL GUIDELINES: CERVICAL CANCER SCREENING
Guideline Source |
|
PHMI Measure |
Cervical Cancer Screening |
Guideline Language |
Age 21-65 (USPSTF):
No Testing (USPSTF):
|
Many key activities in this guide include considerations for utilizing the intervention to improve equitable health outcomes and reduce the effects of racism, bias and discrimination. Key Activity 4: Use a Systematic Approach to Address Inequities within the Population of Focus describes key action steps for how to make an intentional and explicit effort to identify inequities, understand root causes and reduce those inequities.
This guide also offers resources for going deeper into organizational and ecosystem-level work to advance equitable outcomes through Key Activity 18: Strengthen a Culture of Equity. More information about this approach can be found in the PHMI Equity Framework and Approach.
Integrated behavioral health supports are important for adults, as behavioral health support is likely to boost health outcomes and enhance patients' quality of life. One foundational change is to ensure that the care team includes behavioral health staff as core members of the team; this is covered in detail in the Care Teams and Workforce Guide.
We also offer a resource, Pre-Visit Planning: Leveraging the Team to Identify and Address Gaps in Care, that includes recommended behavioral health screenings.
Throughout the key activities in this guide, we have incorporated considerations for providing trauma-informed care in the approach to cancer screening. Trauma is recognized as a potential barrier to seeking or engaging in cancer screening activities. Activities involved in breast, colon and cervical cancer screening may serve as potential triggers associated with the original trauma and should be considered and addressed carefully by clinicians. This guide, therefore, includes a resource for Trauma-Informed Population Health Management. For additional information about managing behavioral health conditions beyond screening, please see the behavioral health implementation guide.
For many key activities in this guide, we have highlighted considerations related to social needs at the individual or population level, such as expanding referral networks. Key Activity 14: Use Social Needs Screening to Inform Patient Treatment Plans can help practices better understand and support patient- and population-level needs. For Medi-Cal patients and families with high levels of social need, such as those experiencing homelessness, referrals to Enhanced Care Management (ECM) and Community Supports programs are available; see Key Activity 17: Provide Care Management for more.
For going deeper in this area, practices can see Key Activity 16: Continue to Develop Referral Relationships and Pathways for common social needs and Key Activity 15: Strengthen Community Partnerships to build upon the strengths, infrastructure and resources available in the community. More information about this dual patient- and population- level approach is available in the PHMI Social Health Framework and Approach.
Our theory of change is that if practices implement the activities contained in this guide, it will lead to improved health and well-being outcomes among the adults served by practices. See Appendix B: Theory of Change for a suggested driver diagram.
Foundational Key Activities
Activities that all practices should implement as part of their cancer screening protocol.
KEY ACTIVITY #1:
Convene a Multidisciplinary Implementation Team for Cancer Screening
This activity involves preparing the practice to address all seven elements of person-centered population-based care: operationalize clinical guidelines; implement condition-specific registries; proactive patient outreach and engagement; pre-visit planning and care gap reduction; care coordination; behavioral health integration; address social needs.
Overview
This activity provides guidance for developing, launching and sustaining the multidisciplinary team within your practice that will be responsible for the planning and implementation of all of the key activities in this guide and overseeing related quality improvement and equity efforts, as outlined in Appendix C: Developing a Robust Measurement Strategy.
The implementation team is so important that it appears first in our sequenced list of key activities. Improving your practice’s key outcomes for each population of focus and reducing equity gaps requires the aligned efforts of all care teams and nearly all functional areas of the practice, not just those working directly with patients.
This team is responsible for ensuring that all key activities in this guide, including those related to screening for social needs, are implemented. As you put together this multidisciplinary implementation team, you should identify a diverse group of staff who are reflective of the community served and who represent the lived experience of patients. In addition to implementing the activity focused on and applying a systematic approach to decrease health inequities, the team should apply an equity lens to every step outlined in this guide to help ensure that any improvements are equitably spread among the patient population. To achieve optimal functioning and impact, all members of this diverse multidisciplinary team should have their perspectives proactively included.
Relevant health information technology (HIT) capabilities to support this activity include care guidelines, registries, clinical decision support, care dashboards and reports, quality reports, outreach and engagement, and care management and care coordination (see Appendix E: Guidance on Technological Interventions). To enable team coordination, thought must be given to how to access relevant technology and how data is consistently captured, can be distributed, integrated into workflows, and how data is accessible across team members. Where possible, it is desirable to avoid duplication of data entry, siloing of information in standalone applications and databases, and the need to work in multiple applications requiring separate logins.
Action steps and roles
1. Develop a time-limited group of leaders within the practice to start this process.
Suggested team member(s) responsible: Chief medical officer or equivalent and office manager or quality improvement coordinator.
Start with a small group of leaders from your practice (some of whom will be on the implementation team) who can help refine the charge or scope of work of the implementation team and both identify and engage the people/roles that will be required to implement the scope of work of the team.
2. Develop a preliminary scope of work or charge outlining the responsibilities of the implementation team.
Suggested team member(s) responsible: Time-limited group of practice leaders.
This scope or charge includes but may not be limited to enabling, aligning, leveraging and supporting the planning and implementation of all foundational key activities in this implementation guide for adults with preventive care needs so that the practice meets the foundational competencies for providing high-quality preventive care.
However, there may be further foundation building work needed at your practice in order for you to succeed at the above key activities. The Population Health Management Capabilities Assessment Tool (PhmCAT) is a multidomain assessment that is used to understand current population health management capabilities of primary care practices. This self-administered tool can help your practice identify opportunities and priorities for improvement.
If your practice has not scored highly in the domains of leadership and culture; the business case for population health management, technology and data infrastructure; or empanelment and access, consider implementing the activities listed in the four guides on Building the Foundation before or in parallel to working on key activities related to adult preventive care.
The multidisciplinary implementation team should include those empowered to make changes in workflows, policies and staff assignments. They should be respected influencers in the organization (early adopters) who can also guide the change management process. They should also include those with expertise in partnering with patients on cancer screenings.
3. Identify leadership and key actors for the implementation team.
Suggested team member(s) responsible: Time-limited group of practice leaders.
- Appoint a “champion” or lead person (e.g., “adult prevention implementation coordinator” to oversee the implementation and coordination of the team.
- Identify key staff who will be the core members of the implementation team. Ensure diversity of position and diversity of gender/race/language. Compensate non-employee members of the team equitably for their time (e.g., patients or community members with lived experience).
- For the adult prevention multidisciplinary implementation team, it is important to include members of the care team, the patient support team, outreach team, social support team, and the electronic health record (EHR) or data team. This could include a core team and an expanded team. Potential members include:
- Adult and family primary care providers (e.g., medical doctor (MD), doctor of osteopathic medicine (DO), advanced practice registered nurse (APRN), or a physician assistant (PA)).
- Registered nurse (RN).
- Medical assistant (MA) or licensed vocational nurse (LVN).
- Social worker.
- Care coordinator.
- Community health worker.
- A member of the information technology (IT) or EHR team, (as part of the expanded team).
- QI lead.
- Billing manager or similar (as part of the expanded team).
- A frontline staff member who interfaces with patients by phone and at check-in.
- Invite identified people to become part of the implementation team and ensure that they have designated time for their participation and/or are compensated equitably for their time.
Teams should engage representation from information technology (IT) to support the work of pulling data from the electronic health record (EHR) and embedding updated data into tracking and evaluation.
4. Launch the implementation team and set it up for success.
Suggested team member(s) responsible: Clinical coordinator, chief operating officer (COO), or chief medical officer (CMO).
This work includes:
- Ensuring that the team understands their charge or scope of work.
- Developing a team charter outlining this work.
- Defining roles and responsibilities including the anticipated commitment (in hours) on a monthly basis. Create a compensation plan for nonemployee members of your team (e.g., patients or community members).
- Establishing a meeting structure, file structure and communications structure to support effective, efficient work.
- Dedicating time and effort to forming, storming, norming and performing as a team. The Team Communication and Working Styles Template is one tool that team members can complete and share with other teammates to accelerate this process.
- Understanding baseline data related to outcomes of interest (e.g., timely postpartum visits and baseline prenatal depression screening), along with data related to known and perceived barriers to these outcomes. Assess stratified outcomes data to identify quality performance disparities in particular subpopulations.
- Prioritizing elements within the scope of work, informed by baseline data and identified population needs
We recommend that practices consider planning and attempting to implement the activities in the sequence provided in this guide, focusing first on the key activities before focusing on the activities suggested under “Going Deeper” or “On the Horizon.” However, different practices may follow different paths toward implementation
5. Develop a simple yet robust measurement strategy and learning system to guide your improvement efforts.
Suggested team member(s) responsible: QI lead or equivalent.
A learning system enables a group of people to come together to share and learn about a particular topic, to build knowledge and speed up improved outcomes. A simple yet robust measurement strategy and learning system:
- Contains a balanced set of measures looking at outcomes, processes and possibly unintended secondary effects (e.g., increased cycle time and impact on team well-being).
- Incorporates the patient perspective and the perspective of staff (front desk and others), care team members, and management.
- Allows the team to determine if the process or system has improved, stayed the same, or gotten worse.
- Helps guide improvement efforts and informs practice operations. See Appendix A: Sample Idealized System Diagram: Weaving Your Measurement Strategy and Learning System into Practice Operations for a sample system diagram for how your measurement strategy can be used to support practice operations.
Your practice should track the core and supplemental measures for colorectal cancer, breast cancer and cervical cancer screening. These can be considered outcome measures because there is ample evidence that improved timely screening rates will improve overall population health outcomes for cancer survival.
In addition to the core and supplemental measures, practices should track process measures and balancing measures. Appendix C: Developing a Robust Measurement Strategy describes and defines the key milestones in the development of a robust measurement strategy, including definitions for each of these terms.
Suggested process measures:
- Percentage of adults who are sent a reminder regarding colorectal cancer screening who initiate screening activity.
- Percentage of adults who are sent a reminder regarding breast cancer screening who initiate screening activity.
- Percentage of adults who are sent a reminder regarding cervical cancer screening who initiate screening activity.
Suggested balancing measures:
- One or more measures related to patient satisfaction.
- One or more measures related to staff satisfaction.
Practices can also look at other metrics to understand the progress of specific improvement initiatives over time. These may include:
- Progress on the Population Health Management Capabilities Assessment Tool (PhmCAT).
- Progress towards foundational competencies listed in this implementation guide. For example, “Yes or No: Did your practice achieve the following foundational competency ‘Provide or Arrange for Cancer Screening?'
- Any other care gaps, clinical guidelines or measures your practice feels are important to prioritize.
Applying an equity lens
Your practice is likely achieving better outcomes with some patients than others. To understand who the practice is achieving poorer adult prevention outcomes for, practices should stratify their data based on race, ethnicity and language (REAL); sexual orientation and gender identity (SOGI); and other patient characteristics (e.g., social needs, etc.) within the Population of Focus. See more in Key Activity 4: Use a Systematic Approach to Address Inequities within the Population of Focus. The ability to segment data in such a manner can lead to profound insights about structural challenges driving some of the health outcomes. The Advancing Equity Through Data Quality and Reporting section of the PHMI Data Quality and Reporting Guide provides more guidance on this.
Putting it all together
We recommend that your practice record your measurement strategy in one place. This Measurement Strategy Tracker contains all the fields we believe are most useful; it can be customized to meet your practice’s needs.
6. Plan and implement regularly scheduled meetings of the implementation team.
Suggested team member(s) responsible: QI lead or equivalent.
- Hold time on team members' calendars for standing meetings. Consider biweekly (twice monthly) meetings to start with. The frequency, duration and focus of these meetings may change as you consider additional populations or subpopulations, additional sites or locations, and the changing nature of the work.
- Develop a system to efficiently report on all work streams and track follow-up items. The Action Plan Template is one tool that can be used to focus your team around the foundational competencies and define responsibility for actions steps to be taken for each project your team has prioritized to work on.
7. Make adjustments based on data from the team’s measurement strategy and feedback loops.
Suggested team member(s) responsible: Multidisciplinary team.
- Review data and feedback at least monthly and adapt efforts as needed. Adaptation could include any or all of the following:
- Amending the charge or scope of work.
- Modifying meetings or meeting structures.
- Changing the team composition (adding or removing members).
- Refining key activities to better meet the needs of patients and practice staff and to improve outcomes or reduce inequities.
- Modifying the measurement strategy and/or feedback loops to better understand what is and isn’t happening.
- On an annual basis, the team’s charter and core membership should be reviewed. As the implementation team's goals are met, the team could disband, meet less frequently (e.g., twice per year), or fold this meeting into a similar standing meeting that occurs separately.
See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
Evidence base for this activity
Pandhi N, Kraft S, Berkson S, Davis S, Kamnetz S, Koslov S, Trowbridge E, Caplan W. Developing primary care teams prepared to improve quality: a mixed-methods evaluation and lessons learned from implementing a microsystems approach. BMC Health Serv Res. 2018 Nov 9;18(1):847. doi: 10.1186/s12913-018-3650-4. PMID: 30413205; PMCID: PMC6230270.
Sarfaty M, Wender R. How to Increase Colorectal Cancer Screening Rates in Practice. CA: A Cancer Journal for Clinicians. 2007 Nov 1;57(6):354–66.
Percac-Lima S, Grant RW, Green AR, Ashburner JM, Gamba G, Oo S, et al. A Culturally Tailored Navigator Program for Colorectal Cancer Screening in a Community Health Center: A Randomized, Controlled Trial. Journal of General Internal Medicine. 2008 Dec 6;24(2):211–7.
Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, et al. Evaluation of Interventions Intended to Increase Colorectal Cancer Screening Rates in the United States. JAMA Internal Medicine. 2018 Dec 1;178(12):1645.
Adams SA, Rohweder CL, Leeman J, Friedman DB, Gizlice Z, Vanderpool RC, et al. Use of Evidence-Based Interventions and Implementation Strategies to Increase Colorectal Cancer Screening in Federally Qualified Health Centers. Journal of Community Health. 2018 May 16;43(6):1044–52.
KEY ACTIVITY #2:
Develop or Update the Practice’s Cancer Screening Protocols
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; implement condition-specific registries; proactive patient outreach and engagement; pre-visit planning and care gap reduction; care coordination.
Overview
This activity provides general guidance for developing or refining a cancer screening protocol that aligns with the PHMI clinical guidelines, establishes clinical and supportive processes that provide a framework for implementing the guidance safely, and leverages the capabilities of appropriate staff to carry out the protocols. It is the foundation of many of the remaining key activities in this implementation guide.
Cancer screening is essential to early detection and treatment of cancers. Aligning a practice’s screening protocols with the relevant guidelines and ensuring a standard team-based approach to identify and address screening status helps to ensure that your practice is able to identify and offer cancer screening to all eligible patients. Early detection and treatment are of critical importance among populations that have a higher incidence of breast, colon and cervical cancer.
The cancer screening protocol includes applying an equity lens to help ensure that barriers to cancer screening are addressed, including economic, social, historical and cultural barriers, and all patients who are eligible for and due for screening, especially those who are at high risk for cancer, receive appropriate screening and follow-up. Key activities in the protocols should be prepared to address relevant social, cultural and linguistic barriers and needs of the population.
Relevant HIT capabilities to support this activity include care guidelines, registries, clinical decision-making support, care dashboards and reports, quality reports, outreach and engagement, and care management and care coordination (See Appendix E: Guidance on Technological Interventions). Reports should have the capacity to filter by provider, location and care team (where applicable).
Access to outside data may be a consideration or requirement (e.g., California Immunization Registry (CAIR) or immunization registry data and data from other practices), as services received outside the health center may be an important part of screening and follow-up. Ideally, this is accomplished by real-time data exchange, but where not possible, it may require manual entry. Reports may need to include not only the EHR but care coordination and population health management applications or freestanding referral registries. While claims data may be helpful in this regard, lag time may impact its usefulness. Patient-facing applications should be strongly considered to promote patient activation by helping assure that patients are informed and appreciative of the nature and importance of recommended care.
Action steps and roles
1. Understand the latest recommendations on who is eligible and needs to be screened for colorectal cancer, breast cancer and/or cervical cancer and how abnormal findings on those screenings should be managed.
Suggested team member(s) responsible: Medical director or their designee.
See the clinical practice guidelines section earlier in this guide. Ensure your practice uses the latest guidelines.
2. Develop or update a colorectal cancer screening protocol for your practice.
Suggested team member(s) responsible: Medical director or their designee.
- In addition to the USPSTF recommendations linked to in step 1, review the National Cancer Institute’s Colorectal Cancer Screening (PDQ ®) - Health Professional Version and the CDC’s Guidance on Colorectal Screening Tests for additional practical guidance on colorectal cancer screening.
- Include a summary statement on colorectal cancer screening, including:
- The evidence base for colorectal cancer screening.
- Benefits of colorectal cancer screening.
- Potential risks of colorectal cancer screening.
- Recent changes to the practice’s colorectal cancer screening protocol, if any.
- Management of abnormal or suspicious findings, including patient contact information.
- The colorectal cancer screening protocol should include the following sections (see clinical practice guidelines)
- Who the screening protocol applies to, including initiation age and cessation age for those at average risk, and high-risk individuals.
- The screening methods available, including (for each method):
- Name and brief description of the screening method.
- Who and when the screening method are indicated for and any exclusionary criteria for this method.
- The known risks and benefits of the screening method.
- Recommended frequency of screening using this method.
- Who – meaning which role(s) within the practice – can initiate and/or provide screening using this method (see also Key Activity 5: Develop and Implement Standing Orders).
- Guidance for patients on using this method and preparing for the test, including the opportunity for them to ask questions and have them answered.
- Guidance on documenting the screening, including the screening method in the patient’s record.
- Guidance and documentation for patient declination. For more information, see Key Activity 8: Refine and Implement a Pre-Visit Planning Process for guidance on documenting when a patient declines.
- Follow-up of abnormal or suspicious findings for each technique.
- Date the protocol was approved for use.
Here is a sample colorectal cancer screening decision tree that your practice can adapt to meet your protocols. that your practice can adapt to meet your protocols.
Preventive healthcare includes participation in select screening tests that have been shown to identify disease early—before symptoms develop—and when treatment is most likely to be effective. This guide addresses steps to support screening for patients at average risk for breast, cervical, and colorectal cancer. Those who are at average risk should be encouraged and supported to undergo screening tests at the frequency noted in the PHMI clinical guidelines.
Some people, however, are at elevated risk for certain types of cancer, and they should be screened using specific screening methodologies at intervals that may differ from routine screening. Those at elevated risk should not be advised to follow the routine screening guidance outlined in the Implementation Guides but should follow individualized screening guidance in accordance with their risk for a particular disease.
Conditions and circumstances that elevate a person’s risk may be related to the presence of signs or symptoms suggestive of cancer, a personal history of cancer or of pre-cancerous conditions, a close family member with a specific cancer or certain types of exposure. When determining if a person is at elevated risk, it is useful to review their health history and ask the following questions at regular intervals, because an individual’s risk status may change over time:
- Have you ever been diagnosed with breast/cervical/colorectal cancer or a precancerous condition?
- Do you have a first degree relative (biological parent or sibling) who has been diagnosed with cancer or a precancerous condition?
- Do you have a genetic marker or syndrome associated with higher cancer risk?
- Do you have any medical conditions that place you at higher risk for cancer (for example, Crohn’s disease increases the risk for CRC cancer)?
- Have you had exposure to drugs or radiation that might affect your risk for cancer?
If a patient is identified as having an elevated risk for breast, cervical, or colorectal cancer, the primary care provider should discuss this risk with the patient and advise the patient of the modified recommendations for persons at elevated risk.
For additional information concerning increased risk for breast cancer, review the following guidance:
- American Cancer Society Recommendations for the Early Detection of Breast Cancer
- US Preventive Services Task Force Full Recommendation for Breast Cancer Screening, Practice Considerations
For additional information concerning increased risk for cervical cancer, review the following guidance:
- US Preventive Services Task Force Cervical Cancer: Screening Recommendations, Clinical Considerations
- Cervical Cancer Screening for individuals at average risk: 2020 guideline update from the American Cancer Society
For additional information concerning increased risk for colorectal cancer, review the following guidance:
- US Preventive Services Task Force Recommendations for Colorectal Cancer: Screening, Practice Considerations
- American Cancer Society Guideline for Colorectal Cancer Screening
Guidance Provided by:
- Margaret Franckhauser, MS, MPH, RN
- Adults with Preventive Health Needs Subject Matter Expert
References:
- U.S. Preventive Services Task Force. U.S. Preventive Services Task Force [Internet]. www.uspreventiveservicestaskforce.org. U.S. Preventive Services Task Force; 2022 [cited 2024 Dec 9]. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/
- American Cancer Society. Cancer Screening Guidelines | Detecting Cancer Early [Internet]. www.cancer.org. American Cancer Society; 2023 [cited 2024 Dec 9]. Available from: https://www.cancer.org/cancer/screening/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html
3. Develop or update a breast cancer screening protocol for your practice.
Suggested team member(s) responsible: Medical director or their designee.
- Practices should consult the guidelines for breast cancer screening in the clinical practice guidelines (hyperlink within document) section earlier in this guide. Practices should also review the National Cancer Institute’s Breast Cancer Screening (PDQ ®) - Health Professional Version, and the CDC’s Breast Cancer Screening Change Package for additional practical guidance on breast cancer screening.
- Include a summary statement on breast cancer screening, including:
- The evidence base for breast cancer screening.
- Benefits of breast cancer screening.
- Potential risks of breast cancer screening.
- Recent changes to the practice’s breast cancer screening protocol, if any.
- The breast cancer screening protocol should include the following sections:
- Who the screening protocol applies to, including initiation and cessation ages, and high-risk individuals.
- The screening method available, including:
- Name and brief description of the screening method (e.g., screening mammography).
- Who the screening method is indicated for and any exclusionary criteria for this method.
- Recommended frequency of screening using this method.
- Who – meaning which role(s) at the practice – can initiate and/or provide screening using this method (see also Key Activity 5: Develop and Implement Standing Orders).
- Guidance for patients on using this method and preparing for the method, including the opportunity for them to ask questions and have them answered.
- Guidance on documenting the screening in the patient’s record.
- Recommended follow-up of abnormal or suspicious findings.
4. Develop or update a cervical cancer screening protocol for your practice.
Suggested team member(s) responsible: Medical director or their designee.
- Practices should consult the guidelines for cervical cancer screening in the clinical practice guidelines section in this guide . Practices should also review the National Cancer Institute’s Cervical Cancer Screening (PDQ ®) - Health Professional Version for additional practical guidance on cervical cancer screening.
- Include a summary statement on cervical cancer screening, including:
- The evidence base for cervical cancer screening.
- Benefits of cervical cancer screening.
- Potential risks of cervical cancer screening.
- Recent changes to the practice’s cervical cancer screening protocol, if any.
- The cervical cancer screening protocol should include the following sections:
- Who the screening protocol applies to, including initiation and cessation ages, and high-risk individuals.
- The Screening Methods available, including (for each method):
- Name and brief description of the screening method.
- Who the screening method is indicated for and any exclusionary criteria for this method.
- Recommended frequency of screening using this method.
- Who – meaning which role(s) at the practice – can initiate and/or provide screening using this method (see also Key Activity 5: Develop and Implement Standing Orders).
- Guidance for patients on using this method, including the opportunity for them to ask questions and have them answered.
- Guidance on documenting the screening in the patient’s record.
- Follow-up of abnormal or suspicious findings.
5. Monitor the cancer screening protocol for accuracy and completeness.
Suggested team member(s) responsible: Quality improvement lead or their designee.
It is critical to have a system to review and update the cancer screening protocols on a periodic basis, as new research often results in refinements, changes and updates of national recommendations. Ideally, at least one clinical member of the implementation team (see Key Activity 1: Convene and Multidisciplinary Implementation Team for Cancer Screening) should be tasked with reviewing the protocols on a periodic basis (six months or 12 months) and bringing suggested revisions before the team for consideration in modifying the protocols.
As of 2024, clinician representatives participate in the PHMI Clinical Guidelines to review and endorse clinical guidelines, including reviewing updated evidence. See the Clinical Practice Guidelines for Key Medi-Cal Populations of Focus for more information.
Other members of the implementation team should serve in a supportive role to bring identified changes forward to the team for review and consideration.
In addition, the implementation team should create a feedback loop with the practice’s care team about any real or potential gaps or errors in the cancer screening protocol. The cancer screening protocol should be modified as needed whenever changes, errors or gaps are discovered.
See also these related key activities:
- Key Activity 3: Use Care Gap Reports or Registries to Identify All Patients Due for Cancer Screening.
- Key Activity 5: Develop and Implement Standing Orders.
- Key Activity 7: Create and Use Clinician Reminders.
- Key Activity 8: Refine and Implement a Pre-Visit Planning Process.
- Key Activity 9: Partner with Patients to Discuss Cancer Screening During Patient Visits.
- Key Activity 11: Provide or Arrange for Cancer Screening.
- Key Activity 12: Develop and Implement a Follow-Up System for Those Who Have Been Screened.
Example workflow
Step 1: A member of the clinical care delivery team (e.g., MD, DO, APRN, PA) is assigned to be a protocol update lead and will scan the literature, review the cancer screening protocols, and bring suggested updates to the implementation team.
Step 2: At each meeting of the implementation team, a protocol update agenda item is addressed. The protocol update lead brings forward any identified gaps, errors or changes in national standards for the team’s consideration.
Step 3: New information, research and national standards (via USPSTF and others) are brought forward for consideration by the protocol update lead and the evidence base is reviewed. Team members suggest changes and discuss how these changes will be operationalized in the protocol.
Step 4: The protocol update lead drafts the suggested protocol changes and presents them to the implementation team for review and finalization. This process may continue until the implementation team is satisfied with the changes and the new protocol.
Step 5: The protocol update lead assumes responsibility for sharing the new protocol with all members of the team involved in implementation.
Implementation tips
- Assign one or two members of the team to receive and follow up on updates from the USPSTF. These updates automatically alert providers when new recommendations are posted and finalized.
- Review any changes in the guidelines with clinicians and clinical support so that discussions with patients reflect the current recommendations.
- Note the availability of choices in certain types of cancer screening, such as colorectal cancer, and inform patients of the differing testing schedule based on the type of test selected.
- Be sensitive to barriers, such as out-of-pocket expenses, transportation needs, and scheduling needs that affect a patient’s ability to carry out the screening.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
Evidence base for this activity
Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, Esener S, Fitzgerald RC, Gambhir SS, Kuhn P, Rebbeck TR, Balasubramanian S. Early detection of cancer. Science. 2022 Mar 18;375(6586):eaay9040. doi: 10.1126/science.aay9040. Epub 2022 Mar 18. PMID: 35298272.
KEY ACTIVITY #3:
Use Care Gap Reports or Registries to Identify All Patients Due for Cancer Screening
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; implement condition-specific registries; address social needs.
Overview
This key activity provides detailed guidance on how to develop and use a continuously updated list of all patients eligible for recommended screenings through a care gap report or registry. Please note that this activity focuses on specific cancer screenings, but many other preventive and maintenance services are needed to provide comprehensive preventive care. See the Pre-Visit Planning: Leveraging the Team to Identify and Address Gaps in Care resource for a more complete list.
Care gaps are gaps between the recommended care that a patient should receive according to clinical guidelines and the care a patient has actually received.
Most EHRs already have a module that identifies what services are due for each patient (see examples below). It is important that clinicians address these services and document efforts to communicate with patients about the screening recommendations, barriers that may affect the patient’s ability to undergo the screening, choices for screening available to the patient, and other relevant information in the EHR. Some patients may have elected to pursue screening through another provider. For example, a person may have obtained a mammogram through an outside provider. Efforts should be made to enter the date of screening into the practice’s EHR and to obtain the results of the screening and enter that information into the EHR through an electronically accessed report or scanning the report.
FIGURE 3, FIGURE 4, FIGURE 5: EXAMPLES OF INDIVIDUALS WITH CANCER SCREENING CARE GAPS
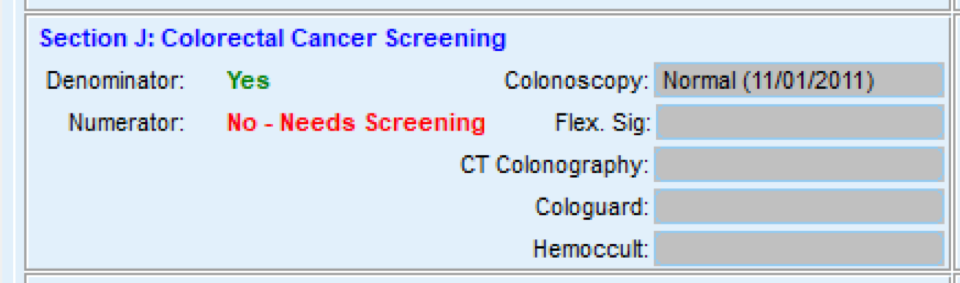
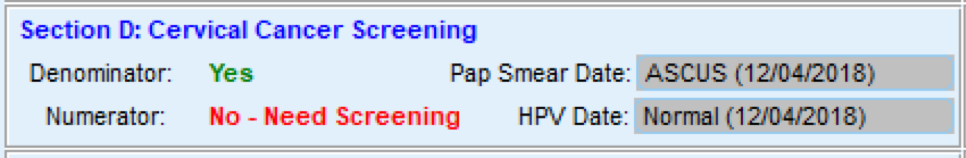
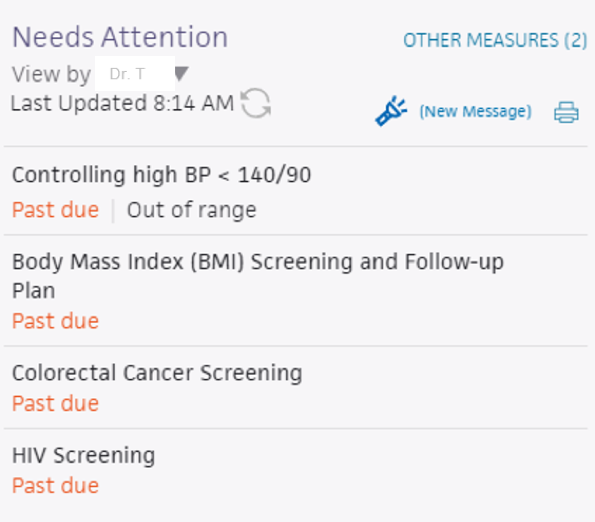
FIGURE 6 AND FIGURE 7: EXAMPLES OF A CARE GAP REPORT.
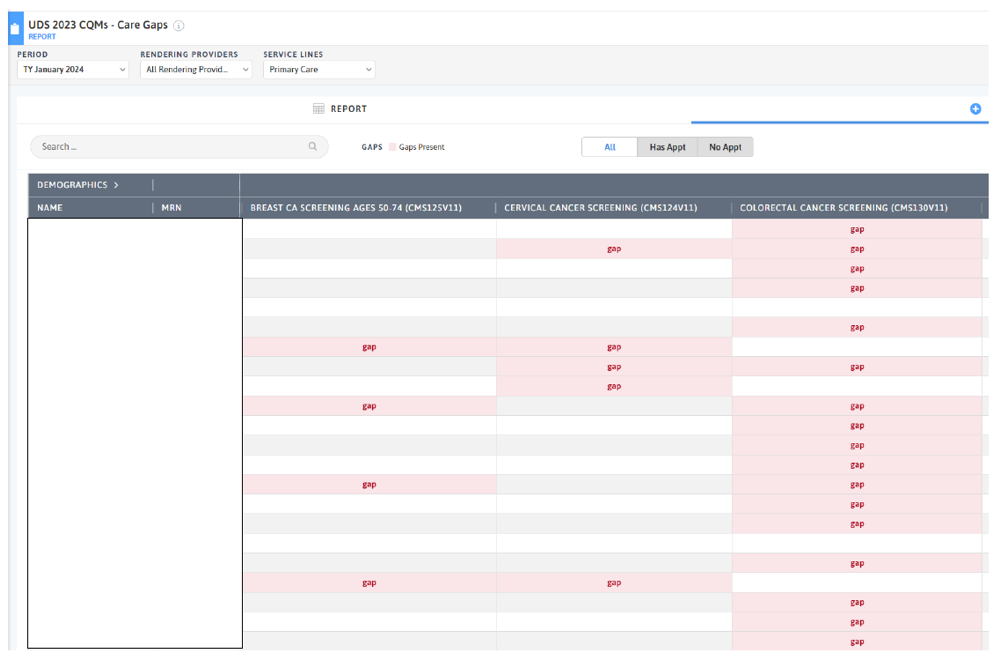
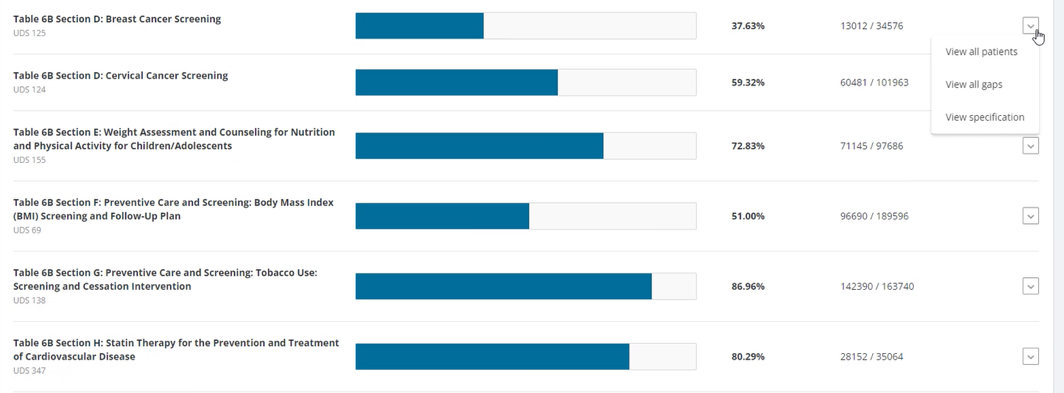
FIGURE 8 AND FIGURE 9: EXAMPLES OF CARE GAP REPORTS FOR A POPULATION FOR COLORECTAL CANCER SCREENING.
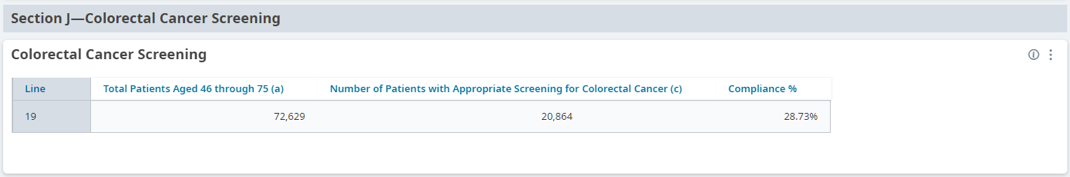
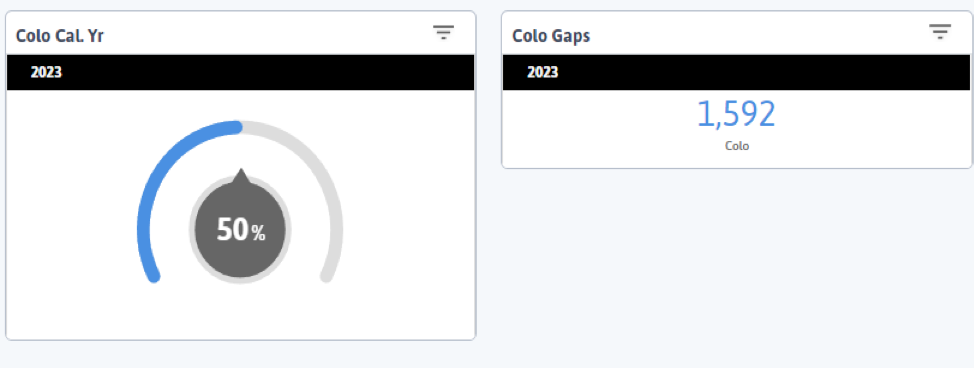
Rather than put the responsibility on the individual care team member for searching through charts or remembering which patients need further preventive care or follow-up, this activity provides guidance for how the practice can efficiently leverage electronic health records (EHRs) for all its patients.
Care gap reports are essential in understanding how well practices are meeting clinical care guidelines for various measures. This awareness supports improvement, consistency and reliability in meeting care guidelines. At the care team level, gap reports focus on due or overdue labs, screenings, or other interventions for patients assigned to your care team. These lists can be used to:
- Support improvements to the pre-visit planning process, develop standing orders, and improve other routine clinical workflows designed to systematically identify and address gaps in care.
- Remind providers of needed orders for a clinical visit.
- Prioritize patients for whom care teams should provide proactive outreach and reminders for engaging in care.
- Support quality improvement efforts with an equity lens.
Actively identifying and acting on care gaps helps to ensure that all eligible patients assigned to your practice receive timely screenings and other preventive services. For adults with preventive health needs, care gap reports can identify patients who are due for regular or infrequent required screenings in accordance with clinical care guidelines, including guidelines that may be established by specific payors.
Many practice patients experience barriers in accessing care due to structural and historical racism, homophobia, xenophobia, and other biases that have historically disadvantaged individuals and groups from receiving equitable services. Defining clear criteria and gaps for patients due for specific screenings or preventive services helps to illuminate groups who have not had equitable access and combats biases by standardizing expectations for who is due for what care, providing a starting point for ensuring reliable and equitable access.
Staff can identify potential barriers to patients accessing and engaging in care by using enhanced care gap reports to filter and display the data alongside demographics, social needs, behavioral health needs, and communication preferences. This information can be used to promote a person-centered approach when designing the care plan and promote self-care and other patient engagement activities, such as conducting outreach in the patients’ preferred language. Furthermore, care gap reports that segment the data into cohorts based on demographic and other personal information may help the team identify inequities in care, access and outcomes, which can inform improvement efforts.
Care gap reports can be used during pre-visit planning to identify people for whom social needs screening has not yet been completed. This creates an opportunity to identify unmet social health needs and connect patients with resources that address their social needs. It also provides an opportunity to identify patients who have undergone screening at another site, obtain screening reports to guide clinical follow up, if needed, and maintain accurate data on patient completion of recommended screening activities.
Many EHRs already have a module that identifies what services are due for each patient, while others do not. Where this functionality is available, it may not be configurable to align with the health center’s specific protocol or be able to incorporate outside data. Other options for developing registries include supplemental applications, population health platforms, and freestanding customized databases that draw data from the EHR and other sources.
Care gap reports may be embedded in electronic health records or made available through other technology channels (see Appendix E: Guidance on Technological Interventions) and are useful both at the individual patient level and aggregated to identify groups of patients to facilitate population level management through registries.
A registry can be thought of as simply a list of patients sharing specific characteristics that can be used for tracking and management. Both care gap reports and registries should have the capacity to segment patients by age, gender, race, ethnicity and language.
Other relevant HIT capabilities to support and relate to this activity include care guidelines, care dashboards and reports, quality reports, outreach and engagement, and care management and care coordination.
See Appendix E: Guidance on Technological Interventions.
Access to outside data may be a consideration or requirement (e.g., CAIR or immunization registry data and data from other practices), as services received outside the health center may be part of compliance. While claims data may be helpful in this regard, lag time may impact its usefulness. Patient-facing applications should be strongly considered to assure they are informed and appreciative of the nature and importance of recommended care. In California, many healthcare organizations are required or have chosen to participate in the California Data Exchange Framework (DxF), which can facilitate data sharing between clinics, managed care plans (MCPs), and other partners.
Action steps and roles
1. Plan the care gap report.
Suggested team member(s) responsible: Panel manager or data analyst. If it is not clear how the report can be produced, this step may involve one or more people from the practice who work on the EHR and, possibly, the EHR vendor.
For adults with preventive care needs, use guidance from your cancer screening protocols to build care gap reports for all patients eligible and due for colorectal cancer, breast cancer and cervical cancer screening. Identify the inclusion criteria for each report, such as age, any exclusion criteria, and factors that make someone high-risk. You can build reports across different risk states, such as identifying those with a higher risk for colorectal cancer because of their family history of colorectal cancer, a personal history of inflammatory bowel disease, or a personal history of colorectal polyps.
Generating a report within the EHR is may be more complicated for colorectal cancer screening because multiple testing options are available to patients, and each test has its own frequency recommendation. It is less complicated for breast and cervical cancer.
For colorectal cancer screening, it is essential to know the type of test last performed to determine the retesting schedule. For example, those who have had a fecal immunochemical test (FIT) test for colorectal cancer are recommended to screen annually, while those who underwent colonoscopy are placed on a 10-year schedule, provided that the test results were negative. In all cases, your cancer screening protocols and the care gap report should be updated to capture the latest recommended frequency for rescreening (see Key Activity 2: Develop or Update the Practice’s Cancer Screening Protocols).
2. Build the report.
Suggested team member(s) responsible: Data analyst.
Determine whether the EHR has an existing report or one that can be modified to fit the inclusion and exclusion criteria. You should talk to staff who are familiar with the electronic record; in some cases, it may be necessary to consult with the EHR vendor to confirm this information and how to run the report.
The care gap format should include:
- Criteria for inclusion and exclusion in the report.
- The overall compliance rate for the care gap being measured.
- All patients eligible for the screening and their addresses and phone numbers.
- The last date the test was performed, if known or if applicable, the previous results, and the type of test used.
- Preferred method of communication (e.g., phone, email, text).
Reports should be able to display and/or disaggregate the data based on:
- Race, ethnicity and language (REAL) as well as sexual orientation and gender identity (SOGI).
- Any known social or behavioral needs.
- Communication preferences or other preferences that would inform the screening modalities offered, such as documented declination of prior screenings.
- Data on any other characteristic, including insurance data, that could pose a barrier to completing screening.
3. Standardize the data format.
Suggested team member(s) responsible: Panel manager or data analyst.
Standardizing the data format and where and how it is entered is critical to ensuring accuracy in the report.
Document how each data element must be entered into the EHR to populate the fields needed for reporting. In some cases, data on completion of screening must be entered by hand (e.g., when the test is performed by a lab that does not communicate with the EHR). Doing this will require a decision on the part of the practice as a whole and may require staff training and reinforcement on an ongoing basis. Where issues or apparent confusion are identified, regular discussion at team huddles or staff meetings will help in maintaining a standard approach.
Tip: Assign responsibility for the initial review of the reports to confirm data integrity.
4. Develop workflows to support improved patient screening completion rates.
Suggested team member(s) responsible: Panel manager and care team.
At the patient level, ensure that the care gap report can be used for or linked with reminders or alerts for clinicians, as well as for sending reminders to adults who need to come into the practice for recommended cancer screening.
Depending on communication preferences that have been expressed by patients, the patient care gap report may be exported to an automated reminder system that can trigger reminders by phone, text, email, or postal mail. Several studies and case studies have indicated that personal calls are more effective, so in the absence of a known patient preference, practices should consider personal calls as a strong option.
For adults with preventive care needs, cancer screening reminders can get particularly complicated. See Key Activity 7: Create and Use Clinician Reminders.
In addition, as part of the practice’s pre-planning process, patient care gaps should be reviewed and flagged as part of the daily huddle. See Key Activity 8: Refine and Implement a Pre-Visit Planning Process for more details.
5. Develop a process for review of gaps at the population level.
Suggested team member(s) responsible: Panel manager.
Set a report frequency to review care gap reports at regular care team meetings or huddles to develop a plan for improvement at the population level. This may include an outreach campaign to build community awareness of the value of screenings and the availability of easily accessed screening services.
6. Monitor the care gap report for accuracy and completeness.
Suggested team member(s) responsible: Panel manager or data analyst.
It is critical to have bidirectional feedback with the practice’s care team about any real or potential errors in the care gap report, such as:
- Errors in how the data is entered compared to what is required under the new standardized data format.
- Patients who are eligible for and due for screening who are missing from the report.
- Patients who have recently been screened who are still listed as due for a screening.
Errors should be investigated through a chart review. If errors in the report specifications are discovered, the care gap report or process for producing the report should be modified. If the issue is incorrect documentation, staff training and reinforcement of documentation standards will be required.
Additional consideration for sustainability: Ensure there is an internal process for updating the criteria included in the EHR for care gap reports as clinical guidelines change.
See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
Evidence base for this activity
Conderino S, Bendik S, Richards TB, Pulgarin C, Chan PY, Townsend J, Lim S, Roberts TR, Thorpe LE. The use of electronic health records to inform cancer surveillance efforts: a scoping review and test of indicators for public health surveillance of cancer prevention and control. BMC Med Inform Decis Mak. 2022 Apr 6;22(1):91. doi: 10.1186/s12911-022-01831-8. PMID: 35387655; PMCID: PMC8985310.
Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009 Feb 23;169(4):364-71.
KEY ACTIVITY #4:
Use a Systematic Approach to Address Inequities within the Population of Focus
This key activity involves all seven elements of person-centered population-based care: operationalize clinical guidelines; implement condition-specific registries; proactive patient outreach and engagement; pre-visit planning and care gap reduction; care coordination; behavioral health integration; address social needs.
Overview
This activity provides guidance for a systematic, evidence-based approach for identifying and then reducing inequities for adults with preventive care needs. It focuses on the first primary driver in PHMI’s equity framework: Reduce inequities for populations of focus.
FIGURE 10: PHMI DRIVER DIAGRAM
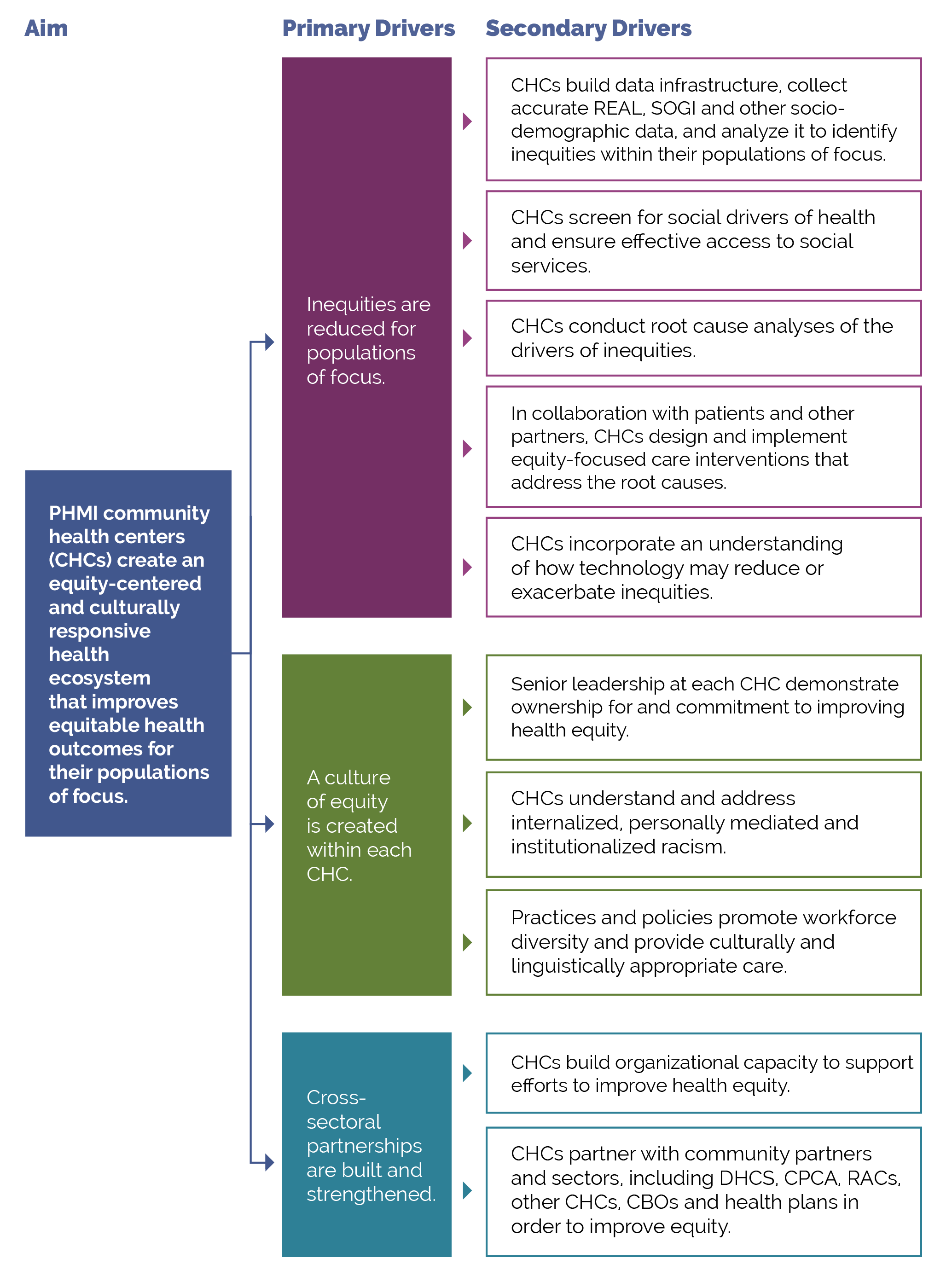
Your practice likely achieves better outcomes with certain populations or subpopulations and worse outcomes with others. Inequitable outcomes are often the result of social and cultural factors that serve as barriers to accessing care and are generally most acute among persons of color, immigrants, persons speaking a primary language other than English, and other populations who have been marginalized. As we work to reduce and eliminate inequitable health outcomes over time, we need to understand both what contributes to these different outcomes, as well as factors that do not contribute to them.
This includes recognizing that race is a social construct determined by society’s perception. While some conditions are more common among people of certain heritage, inequities in conditions, such as cancer, diabetes, and adverse maternal outcomes, have no genetic basis. While genetics do not play a role in these inequitable outcomes, the extent to which inequities in the quality of care received by people of color contribute to inequitable health outcomes has been extensively documented[3]. These inequities are often a direct result of racism, particularly institutionalized racism – that is, the differential access to the goods, services, and opportunities of a society by race[4]. Racial health inequities are evidence that the social categories of race and ethnicity have biological consequences due to the impact of racism and social inequality on people’s health[5]. It is also critical to recognize that we have policies, systems and procedures that unintentionally cause inequitable outcomes for racial, ethnic, language and other minorities in spite of our genuine intentions to provide equitable care and produce equitable health outcomes.
Improving your practice’s key outcomes for each population of focus requires a systematic approach to identifying equity gaps (e.g., who your practice is not yet achieving equitable outcomes for) and then using quality improvement (QI), collaborative design, systems thinking and related methods to reduce these equity gaps.
Identifying and meeting patients’ social health needs is a key driver of reducing inequitable health outcomes. We provide additional guidance in this activity on how to both reduce inequities and meet patients’ social health needs.
Access to data required to identify and monitor inequities, as outlined in the key actions outlined below, is fundamental to this activity.
EHRs have the ability to capture basic underlying socioeconomic, SOGI and social needs-related data but may in some cases lack granularity or nuances that may be important for subpopulations. Mismatches between how the Uniform Data System (UDS) captures REAL data versus how EHRs capture or MCPs report data can also create challenges. This may require using workarounds to capture these details. It is also important to ensure that other applications in use that may have separate patient registration processes are aligned. Furthermore, tracking inequities in accessing services not provided by the health center may also require attention to data sources or applications outside the EHR.
Health centers should also be alert to the potential for technology as a contributor to inequities. For example, patient access to telehealth services from your practice may be limited by the inequitable distribution of broadband networks and patient financial resources (e.g., for phones, tablets, and cellular data plans). EHR-embedded algorithms used to risk stratify populations may also contain inherent racial biases. The Techquity framework can be a useful way to structure an approach to assure that technology promotes rather than exacerbates disparity.
Language, literacy levels, technology access and technology literacy should also be considered and assessed against the populations served at the health center.
Finally, attention needs to be paid to use of best practices in collecting the data, accurate categories in the technologies in which it is collected and stored, and adequate training and monitoring of staff responsible in order to ensure that reports and analysis have a valid basis.
Relevant HIT capabilities to support this activity include care guidelines, registries, clinical decision support (e.g., modifications required to consider disparate groups), care dashboards and reports, quality reports, outreach and engagement, and care management and care coordination see Appendix E: Guidance on Technological Interventions.
Action steps and roles
1. Build the data infrastructure needed to accurately collect REAL, SOGI, social needs and other demographic data.
Suggested team member(s) responsible: Data analyst.
- Race, ethnicity and language (REAL).
- Sexual orientation and gender identity (SOGI).
The PHMI Data Quality and Reporting Guide provides guidance and several resources for collecting this information. According to this guide, the initial step in addressing disparities is to collect high-quality data that fosters a holistic view of patient characteristics and needs. This entails incorporating REAL data, demographic data (age, SOGI, geography) and social needs data. By collecting and monitoring this information, healthcare practices can gain valuable insights into disparities in access, continuity and health outcomes. Steps two to four below provide more information on this process.
Collecting REAL information allows for practices to identify and measure disparities in care while also ensuring that practices are able to interact successfully with patients. This is done by understanding patients’ unique culture and language preferences.[6] KHA Quality has a toolbox that assists with REAL data collection.
The Uniform Data System (UDS) Health Center Data Reporting Guidance (2023 Manual) provides detailed guidance on REAL and SOGI. While UDS does not currently require that practices report on the preferred language of each patient, practices should make an effort to identify and record each patient’s preferred language due to UDS reporting still requiring for languages other than English to be reported.
Accurate data collection requires appropriate fields and options in the EHR and other employed technologies, as well as appropriate human workflows in collecting the data. Staff responsible for data collection should be continuously trained and assessed for best practices in data collection, including promotion of patient self-report.
In addition, practices should work to ensure that patients understand the importance and use of this information to help them feel comfortable and support its collection. High rates of “undetermined” or “declined” responses in these fields may be indicative of the need to attend to these staff training needs.
Collecting this data is important, especially to obtain a complete picture of health for patients who identify as transgender. There are certain risks and condition indicators that are gender-specific, which impact how providers provide care to a patient. This should also be taken into account for cancer screenings for patients who have transitioned but carry the risk of certain cancers based upon their gender at birth and degree of transitioning (e.g., a transgender male who still has a cervix may still be at risk for cervical cancer). By understanding the needs of patients more fully, providers can make more informed decisions for the best treatment of their patients. The World Professional Association for Transgender Health (WPATH) has provided further guidance regarding standards of care related to gender diversity.
2. Use the practice’s electronic health record (EHR) and/or population health management tool to understand inequitable health outcomes at your practice by stratifying your data.
Suggested team member(s) responsible: Data analyst.
This includes reviewing your care gap report or care registry and being able to stratify all of the following:
- Core measures for the population of focus.
- Supplemental measures for the population of focus.
- Process measures for the population of focus.
Stratify this data by:
- Race, ethnicity and language (REAL).
- Sexual orientation and gender identity (SOGI).
- Other factors that can help identify subpopulations in need of focused intervention to reduce an equity gap (e.g., immigrants, people experiencing homelessness, literacy levels, etc.).
FIGURE 11: EXAMPLE OF DATA STRATIFIED BY RACE AND ETHNICITY.
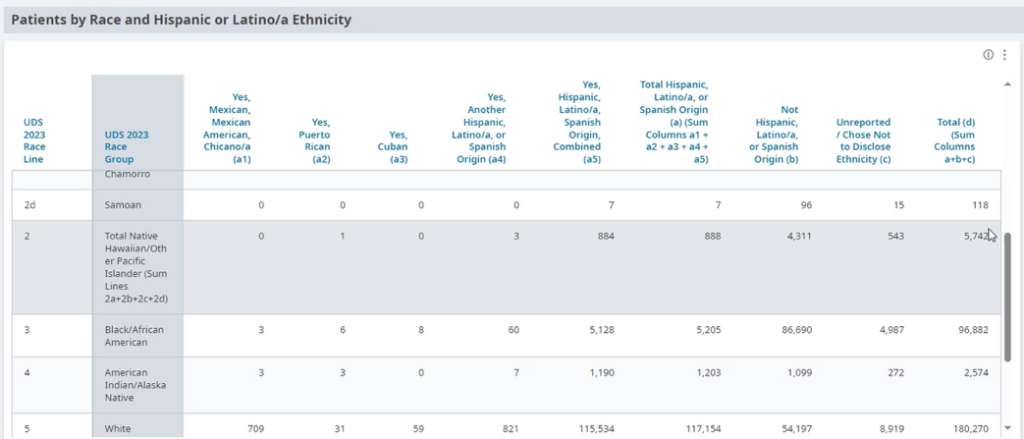
This is not a one-time event, but rather a continuous process (see step 13 below) and should be done in tandem with step three below.
Each practice should define the frequency of review and use of their registry to stratify data for adult preventive care. In early use, the stratified data will support the identification of areas of inequity and allow for prioritization of interventions.
3. Screen patients for social needs.
Suggested team member(s) responsible: Care team.
Key Activity 14: Use Social Needs Screening to Inform Patient Treatment Plans provides guidance on screening patients for health-related social needs and how the information can begin to be used to inform patient treatment plans, including referral to community-based services.
This is not a one-time event, but rather a continuous process and should be done in tandem with step two above.
4. Analyze the stratified data from steps two and three to identify patterns in inequitable outcomes within the population of focus.
Suggested team member(s) responsible: Data analyst
This includes:
- Using data tools to visualize and understand disparities across different populations or subpopulations.
- Data over time (using run charts).
- Exploring trends, patterns and significant differences to understand which demographic groups will require a focused effort to close equity gaps.
This is not a one-time event, but rather a continuous process (see step 13 below) and should be done in tandem with step two (above) and step four (below).
Periodic review of the stratified data allows for the recognition of gap closures and the emergence of new disparities.
5. Conduct a root cause analysis for each population or demographic group that the practice does not yet have equitable outcomes for.
Suggested team member(s) responsible: Multidisciplinary team.
Select root cause analysis approaches that work best for the equity gap you are closing:
- Engage and gather information from patients affected by the health outcome in your root cause analysis (see step six).
- Brainstorming.
- Systems thinking (understanding how interconnected social, economic, cultural and healthcare access factors may be impacting the health outcome).
- Tools that rank root causes by their impact and the feasibility of addressing them (e.g., prioritization matrix and/or an impact effort matrix)
- Visual mapping of root causes and effects (e.g., fishbone diagram).
- Perform focused investigations into selected root causes and gather qualitative data through interviews, surveys or focus groups with the subpopulation of focus.
Present the findings to a broader group of stakeholders, including affected community members, to validate the identified root causes and gain additional insights. Incorporate their feedback and refine the analysis, as needed.
6. Partner with patients to develop additional insights that will help you develop successful strategies.
Suggested team member(s) responsible: Care team and people with lived experience (e.g., patients who are representative of the population(s) of focus).
Using one or more human-centered design methods such as focus groups, 7- Stories, Journey mapping, etc. (see links to these methods below), work with members of each population of focus to better understand their:
- Assets, needs and preferences, as they relate to adult prevention recommendations.
- Cultural beliefs, including traditional healing practices that may impact their understanding of or willingness to engage in certain adult prevention practices.
- Beliefs and level of trust in healthcare generally and in the topic of focus specifically (e.g., cancer screening, immunizations, behavioral health, etc.)
- Barriers to accessing care, including health insurance, transportation, childcare, housing access and other social factors.
- Barriers to remaining engaged in care, including the above-noted barriers, and including cultural beliefs, trust, and concerns about follow-up requirements.
- Trusted sources of information and communication mechanisms and preferences for this population.
- Ideas for improving health outcomes as they relate to the uptake of preventive care.
The patients you partner with for this and other steps in this activity may be part of a formal or informal patient group and/or identified and engaged specifically for this equity work.
7. Identify key activities that address or partially address the identified equity gaps.
Suggested team member(s) responsible: Care team
Based upon the insights your practice has developed for a population of focus and your root cause analysis, determine which of the key activities could address or partially address the equity gap.
Most of the key activities either explicitly address an equity challenge or can be adapted to better address an equity challenge. Examples of key activities that can be adapted to reduce identified equity gaps include but are not limited to:
- Key Activity 6: Conduct Proactive Outreach to Patients Due for Screening.
- Key Activity 13: Coordinate Care.
- Key Activity 14: Use Social Needs Screening to Inform Patient Treatment Plans.
- Key Activity 15: Strengthen Community Partnerships.
- Key Activity 16: Continue to Develop Referral Relationships and Pathways.
8. Develop new strategies and ideas to address the identified equity gaps.
Suggested team member(s) responsible: Care team and people with lived experience.
If one or more of your root causes cannot be addressed fully through key activities, use one or more human-centered design methods (see resources below), to develop ideas to improve health outcomes and reduce inequities among adults with preventive care needs.
Developing these ideas is best done with representatives of the population of focus, as they have expertise and experience that may be missing from the practice’s care team. Compensating these patients and community members for time spent on improvement activities is a best practice. During this brainstorm, you are developing ideas without immediate judgment of the ideas in an effort to generate dozens of potentially viable ideas.
Selected resources on human-centered design and collaborative design:
- Center for Care Innovations (CCI) Human-Centered Design Curriculum.
- IDEO’s Field Guide to Human-Centered Design.
- IDEO’s Design Kit: Methods.
9. Determine which strategies to test first.
Suggested team member(s) responsible: Care team and people with lived experience.
Steps seven and eight above help your practice identify existing key activities and generate new ideas. Your practice likely doesn’t have the bandwidth to test all of them, at least not at the same time, so now is the time to prioritize a few to begin testing.
There are many ways to prioritize ideas. The Institute for Healthcare Improvement (IHI) often recommends a prioritization matrix and/or an impact effort matrix.
If you have organized your key activities and new ideas into themes or categories, you may choose to work on one category or select one to two ideas per category to work on.
The number of key activities and/or new ideas that you prioritize for testing first should be based on the team’s bandwidth to engage in testing. So, it is critical to determine the bandwidth for the team(s) who will be doing the testing so that you can determine how many ideas to test first.
10. Use quality improvement (QI) methods to begin testing your prioritized key activities and new ideas for and with the population of focus.
Suggested team member(s) responsible: Care team and people with lived experience.
Nearly all of the key activities and all of your new ideas will require some degree of adaptation for use within your practice and to be culturally relevant and appropriate to your population(s) of focus.
Use plan-do-study-act (PDSA) cycles as feasible, generally starting as small as possible – think “ones” (e.g., one clinician, one hour, one patient, etc.) – and become larger as your degree of belief in the intervention grows.
Whenever testing an activity or new idea, we recommend that the practice:
- Use plan-do-study-act (PDSA) cycles to test your ideas and bring them to scale. See more information on PDSAs below in the tips and resources section.
- Generally, start with smaller scale tests (e.g., test with one patient, for one afternoon, in a mailing to 10 patients, etc.). Use Figure 12: How Big Should My Test Be?, in the tips and resources section to help you decide what size test is most appropriate.
Develop or refine your learning and measurement system for the ideas you are testing. A simple, yet robust learning and measurement system will help you know if your ideas are having their intended effect, if they are having unintended secondary effects (e.g., staff burnout), and how the overall implementation process is going. Consider the following:
- What core measures, supplemental measures and/or process measures is this intervention designed to improve equity for?
- What are or will be the first or early indications that the intervention or idea is working?
- How will you know how the implementation itself is going from a patient perspective, a staff perspective, and a management perspective?
- What are some balancing measures to monitor for the implementation itself and for the time and resources spent on implementation?
- Study the successes and challenges of the test. When feasible, this should include getting feedback from patients directly after testing a new idea with them and incorporating their feedback into your next test.
- Refining the idea, as needed, based on the test.
- Then testing again, increasing on the scale of the test as these tests result in few challenges and better results.
By working out the inevitable kinks in the idea you are testing before taking it to full scale, the practice will make the idea work better for patients and be less frustrating to the care team. Testing and refining also can eliminate the workarounds that occur when a process or approach doesn’t fit well into the system or workflow it is being placed into.
Select resources on quality improvement (QI):
- Institute for Healthcare Improvement (IHI) QI Essentials Tool Kit.
- Cause and effect diagrams.
- Driver diagrams.
- Failure modes and effects analysis.
- Flowcharts.
- Histograms.
- Pareto charts.
- PDSA worksheets.
- Project planning forms.
- Run charts.
- Scatter diagrams.
- IHI’s Videos on the Model for Improvement (Part one and Part two).
- Rapid Experiments to Improve Healthcare Delivery.
- CCI’s ABC's of QI via CCI Academy.
11. Implement – meaning, bring to full scale and sustain – those practices that have proven effective.
Suggested team member(s) responsible: Care team
Once an idea has been well tested and shown to be effective on a small scale, it is time for your practice to hardwire the idea, approach or practice into your daily work. Consider using the MOCHA Implementation Planning Worksheet to think through:
- Measurement.
- Ownership.
- Communication, including training.
- Hardwiring the practice.
- Assessment of workload.
Sometimes, implementation may require that you update your protocol and/or policies and procedures for the populations of focus.
12. Once you have tested, refined and scaled up the initially prioritized ideas, begin testing other ideas.
Suggested team member(s) responsible: Care team and people with lived experience.
Consider revisiting the ideas that were developed previously but not were not initially prioritized for implementation. You might also move through the testing steps above to develop and prioritize new ideas or adapt ideas to better serve additional subpopulations of focus.
13. Put in place formal and informal feedback loops with patients and the care team.
Suggested team member(s) responsible: Care team.
To help ensure that your practice’s ideas are meeting the needs of patients and their families, helping to reduce identified equity gaps and increase the feasibility and sustainability of your practices, it is important to have both formal and informal feedback loops.
For patients, feedback loops could be created using many of the human-centered design tools used to design your improvement activity (e.g., surveys, interviews, focus groups).
Consider establishing a standing funded patient advisory board that is available to design, implement and evaluate all of your practice’s improvement activities.
For the care team, feedback loops might include:
- Existing or new staff satisfaction and feedback mechanisms.
- Regularly scheduled meetings and calls to get staff feedback on processes, methods and tools.
14. Continually analyze your data to determine if your efforts are closing equity gaps.
Suggested team member(s) responsible: Care team.
This includes regular (at least monthly) review of the stratified measures for all of the following:
- Core measures for the population of focus.
- Supplemental measures for the population of focus.
- Process measures for the population of focus.
- Social needs data.
- Any additional measures collected as part of your testing and refinement effort.
Share the data with patients to both show your work to decrease known equity gaps and to solicit ideas for closing them.
Implemenation tips
- When testing a change idea (either a key activity or new idea) for your practice to address a known equity gap, the size of your test is critical.
- We recommend starting with a very small test (e.g., with one patient or with one clinician) or a small test (e.g., with all patients seen during a three-hour period by this clinician) unless you are certain the change idea will lead to improvement. The cost of a failed small test is extremely low and staff will often be excited to test the change idea.
- As you learn what is or isn’t working from each test, you can conduct larger scale tests and tests under a variety of conditions. While at first glance this would appear to slow down the implementation effort, starting small and working out the challenges as you progressively work to full scale actually saves time and resources and is much less frustrating for your patients and care team. The visualization below provides guidance on how big your test should be.
FIGURE 12: HOW BIG SHOULD MY TEST BE?
Resources
KEY ACTIVITY #5:
Develop and Implement Standing Orders
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; pre-visit planning and care gap reduction.
A standing order – a preapproved provider order to perform a specific intervention for any patient who meets the criteria for the order – is used when the step in the workflow specifically requires the order of a qualified provider. An important characteristic of a standing order is that all the patients who meet the criteria for the order receive the same treatment, thereby embedding equity into the clinical approach. Standing orders describe the action to be taken and identify who on the care team is authorized to complete the order. Standing orders are designed to enable care team members other than the primary care provider (PCP) to initiate specific clinical actions to provide timely screening and care, provided that specific criteria are met. Common standing orders include preventive measures such as immunizations, cancer screenings, and other screenings to be conducted ahead of the clinician’s time with the patient. While California does not require a provider order to obtain a screening mammography, the FDA requires that Nevertheless, health centers may provide their patients with a referral that specifies the name and address of the primary care clinician requesting the test.
To limit the potential for errors and ensure patient safety, standing orders should be carefully designed, regularly reviewed, and revised as necessary. They should be based on evidence-based recommendations and best practices, and they should have broad support from the medical director, practice manager, physicians, and other staff. In addition, they should outline the criteria for initiating, modifying or discontinuing a particular course of action or treatment for a patient[7].
Standing orders, in concert with other key activities, allow care team members to work to the full scope of their license and provide scaffolding to support care team members as they work together to support patient care. They promote practice workflow efficiency and effectiveness by standardizing routine care, thus freeing up time for the ordering clinician.
Standing orders help promote equity by ensuring that every patient who meets the criteria for an intervention under the standing order is provided with appropriate services, limiting variation that could occur in interpersonal encounters. Standing orders are particularly useful for preventive care and screening for chronic diseases and conditions such as diabetes, hypertension, cardiovascular disease and cancer, which disproportionately affect certain groups.
Most EHRs have the ability to enable the creation of order sets that can be utilized to create standing orders. Order sets are particularly effective for managing a group of standard adult screening orders for a population. (See Appendix E: Guidance on Technological Interventions for more details on implementing order sets in a commercial EHR). Depending upon the health center’s workflows, these order sets can be placed on the chart by providers or can be drawn down by responsible designated staff according to protocols.
Relevant HIT capabilities to support this activity include electronic access to care guidelines, registries, care gap reports and clinical decision-making support.
Effectiveness of standing orders can be tracked through registries and care quality reports.
Action steps and roles
The steps below outline the actions required to create standing orders. They are adapted from the University of California, San Francisco (UCSF) Center for Excellence in Primary Care (CEPC).[8]
1. Review and understand the latest clinical guidelines for the required standing order and your practice’s already established protocols.
Suggested team member(s) responsible: Quality improvement lead; panel manager, data analyst, medical director or equivalent.
The University of California, San Francisco’s Center for Excellence in Primary Care provides guidance on developing standing orders, along with examples of standing orders for several clinical conditions.
See the clinical practice guidelines section earlier in this guide.
- Note: Regardless of the specific guidelines being used by the practice, the care team should monitor the website containing these guidelines at least annually and/or upon notification of changes to ensure that the latest recommendations are being used by the practice.
- Note: Because California does not require provider orders for screening mammography but does suggest provider referral, the care team should consider whether and how they wish to initiate screening mammography orders. Assisting patients to schedule a mammography supports their ability to carry out the screening plan.
2. Translate the appropriate clinical guidelines into a standing order.
Suggested team member(s) responsible: Panel manager.
The standing order will follow the relevant clinical guidelines and be updated whenever clinical guidelines are updated. It will generally allow the following:
- Registered nurses (RNs) and/or medical assistants (MAs) with proper training may initiate the agreed upon standing order process when patients meet the agreed upon criteria for initiating screening, and do not have any clinical history that may require a clinician to address the concern.
- This may include initiating an order, initiating a referral, or notifying the PCP of the need for screening and/or testing.
In addition to the written standing order, the practice should develop a process map or update other documentation outlining how and when the standing order is to be implemented in the practice’s current workflows.
3. Obtain approval for standing orders from clinical leadership.
Suggested team member(s) responsible: Panel manager and medical director or equivalent.
For the standing order to be valid and in effect, it must be approved by clinical leadership at the practice, signed by a designated valid signatory (e.g., medical director or other physician), and dated, and it must include the effective date for the standing order. Practices should consider including an expiration date and flagging this date in their system to help ensure that the standing order is reviewed and updated regularly. As the clinical guidelines change or other components are updated, the standing order should be signed and dated again by the appropriate party with an effective date for the revision.
4. Train practice staff on how to use standing orders and include this training in clinical onboarding of new staff.
Suggested team member(s) responsible: Medical director or equivalent.
It is critical that practice staff, both those directly named in the standing order and other members of the care team, receive training on the use of this standing order. Such training should include a thorough review of the written standing order and ensure that practice staff understand all of its aspects, including the associated workflow and any additional materials related to the workflow, such as patient education or instructional materials that include nuances that the care team will need to understand.
Training should be provided on each standing order at least annually, retraining should be provided before the effective date on any revisions, and the training should be part of the orientation for all new members of the practice care team.
Based on feedback from the staff, the standing order can be refined to make the instructions clearer (e.g., during training several staff members were confused by a specific instruction, indicating that the instruction needed to be revised).
5. Institute mechanisms to ensure the proper use of the standing order and its effectiveness.
Suggested team member(s) responsible: Clinical director or equivalent or their designee.
It is important that practice team members understand the purpose, scope and limits of standing orders and be ready to implement them appropriately and within legal constraints of professional practice and of the state of California. In some cases, portions of the standing order will be carried out by professionals outside the practice (e.g., mammography center for breast cancer screening or surgical center for colonoscopy). In the case of these standing orders, it may be helpful for the practice to share the orders with the entity carrying out the order so that results are appropriately communicated to the practice in a timely fashion.
It is possible that one or more aspects of the standing order will not work as planned. For example, the practice’s workflow may not fully support the standing order, the wording may be confusing to one or more staff members, or the standing order’s protocol may not be regularly followed exactly (e.g., staff are using workarounds). The practice should routinely check for these and other common challenges and revise and update the standing order, as needed, to ensure that it works for the practice and meets all applicable regulations and guidelines.
Implementation tips
- The same basic workflow for other standing orders that your practice already has in place can be used as a jumping-off point when developing a new standing order. Before beginning work on a new standing order, determine whether your practice has existing standing orders that you can draw from as you develop this one.
- Verify if carrying out standing orders falls within the legal scope of practice for the supportive clinical staff intended to carry them out.
- The first two to four weeks of implementing a specific standing order should be used to test and refine it. We recommend that the practice start with smaller scale tests (e.g., test for one day), study the successes and challenges (including errors) of the test, refine the standing order and/or training on it, as needed, and then test again, increasing the scale of the test as they result in fewer challenges or errors. While this approach may slightly slow down the full implementation of the standing order, by working out the inevitable kinks in the process before taking it to full scale, the practice will make the process safer for patients and less frustrating to the care team. Testing and refining can also eliminate the workarounds that occur when a policy doesn’t fit well into the system or workflow it is being placed into.
- Note that California does not require a practitioner’s order to obtain a screening mammogram, but it does require that the mammography provider inform the patient and the patient’s healthcare provider. Therefore, the standing order should note the procedure for contacting patients. Additionally, practices may wish to share these orders with local imaging centers to facilitate timely and accurate communication of results.
- Common pitfalls of standing orders:
- Standing orders are not updated when screening guidelines are revised and reflect an outdated practice. The remedy is to assign the task of updating the protocols to a member of the team who is responsible for at least annual review and modification.
- New staff are not instructed on the clinical protocols. The remedy is to include review of standing orders as a core element of orientation for all roles named in the standing order.
- Standing orders include the signature of a clinician who is no longer with the practice. The remedy is to review standing orders at least annually and any time a clinician responsible for setting the order has changed roles.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Evidence base for this activity
Alberti LR, Garcia DP, Coelho DL, De Lima DC, Petroianu A. How to improve colon cancer screening rates. World J Gastrointest Oncol. 2015 Dec 15;7(12):484-91.
Ishida K, Weiss E, Kee SA, Yingling CT. Increasing colorectal cancer screening orders using unlicensed assistive personnel. BMJ Open Qual. 2019 Jun 29;8(2):e000545.
Percefull J, Butler J. Improving mammography through effective screening, brief intervention, and referral to treatment at a rural health center. J Am Assoc Nurse Pract. 2020 Jan 16;33(4):324-330. doi: 10.1097/JXX.0000000000000356. PMID: 31972786.
Resources
KEY ACTIVITY #6:
Conduct Proactive Outreach to Patients Due for Screening
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; proactive patient outreach and engagement.
Overview
This activity provides guidance on how practices can develop and use patient reminders to increase the percentage of patients who attend preventive care visits and complete recommended preventive care measures. This includes guidance on both the communication methods (e.g., phone, text, email, postal mail) and tailoring the content of the reminders to meet the needs of your practice’s patients. Conducting proactive outreach can ensure patients know about their recommended cancer screening and are able to access all recommended cancer screenings. Proactive outreach involves identifying and understanding subpopulations who may benefit from outreach, implementing reminders and touches for those populations, and cultivating a trusting and engaging environment.
This should include developing distinct strategies for three populations:
- Patients who are regularly seen by the practice for other health-related reasons (active patients).
- Patients who have been seen by the practice in the past but not over the last 12 months (inactive patients).
- People who are on the practice’s panel but have not been seen by the practice (empaneled, but not yet seen patients).
Proactive outreach is one way a practice can help to detect precancerous and cancerous conditions early. Culturally appropriate proactive outreach enhances early detection of colorectal, breast and cervical cancer. This improves survival rates and enhances patients' quality of life. Proactive outreach helps patients access all the recommended cancer screenings in accordance with your practice’s cancer screening guidelines, which can identify precancerous conditions and provide an opportunity for your practice to intervene before cancer develops.
This approach supports patients and families and boosts participation in preventive care. Numerous studies have demonstrated the effectiveness of culturally tailored patient reminders in helping ensure patients receive recommended cancer screenings.[9][10][11][12]
Practices can focus on equity by using data to identify which populations, subpopulations or groups the current outreach and education efforts don't reach. See the PHMI Data Quality and Reporting Guide the for more information.
Collaboratively designing outreach strategies with community partners and current patients promotes cultural humility and sensitivity. Tailored outreach with personalized reminders addresses diverse needs, reducing inequities in access to care for chronic conditions.
Patient reminders should be tailored to the needs, preferences, culture, and likely barriers to screening of each patient and use their preferred method of communication, when feasible. This activity provides guidance for better tailoring reminders for several historically marginalized populations.
Unmet social needs (e.g., income insecurity, lack of transportation, and health illiteracy) can result in lower cancer screening rates. Proactive outreach, coupled with awareness of health-related social needs, can connect patients to community resources and build trust in the care team.
Which patients are eligible can be found in your practice’s care gap reports for colorectal cancer screening, breast cancer screening and cervical cancer screening. See Key Activity 3: Use Care Gap Reports or Registries to Identify All Patients Due for Cancer Screening for more details.
For high-risk patients or patients with complex health and social needs, consider care management as a more intensive approach for facilitating ongoing communication and coordination. Patient reminders should include information about common barriers to screening and how your practice can address them and, as your patient reminder system becomes more mature, can include more tailored messages for patients with known social needs.
This activity relies on similar capabilities as care gap management, utilizing population views and registries to track adult preventive healthcare gaps against screening guidelines. These registries can be utilized to generate outreach lists for appointment schedulers and/or care managers and other care team members who might be tasked with contacting patients due for services. Many EHRs are capable of storing next-appointment-data, which can also be used to generate lists and may link to automated appointment outreach workflows. Patient-facing outreach and engagement technologies can be utilized to deliver appointment reminders and for patient self-scheduling. Care managers might use care management applications to track and prompt adult preventive health needs, as well as wellness visits.
Other relevant HIT capabilities to support this activity include care guidelines, clinical decision-making support, including patient-facing clinical decision support, care dashboards, and reports. Some health centers may focus extra resources on adult patients identified as high-risk through risk stratification algorithms.
Action steps and roles
1. Start by understanding your practice’s current reminder processes, content, methods and effectiveness (if any).
Suggested team member(s) responsible: Panel manager, data analyst or EHR data manager and multidisciplinary team for cancer screening.
More specifically, determine:
- What reminders, if any, your practice sends out to patients who are eligible and due for colorectal, breast and cervical cancer screening.
- What is the content and languages your practice uses to send cancer screening reminders to patients (if applicable) for initial screening and follow-up.
- How and what communication methods does your practice use, including automated methods.
- How often (e.g., with what frequency) does your practice send out reminders?
- What types of reminders does your practice send including mailed reminders, postcards, letters, and electronic reminders (e.g., text, email, phone, and EHR portal).
- What tailored content, if any, does your practice have for specific tests and specific populations (e.g., Black women, Native Americans, immigrants, patients who are uninsured or underinsured, etc.)
2. Understand the patient population eligible for colorectal cancer, breast cancer and cervical cancer screening at your practice.
Suggested team member(s) responsible: Panel manager.
This information can be found in your practice’s care gap reports for colorectal cancer screening, breast cancer screening and cervical cancer screening. See Key Activity 3: Use Care Gap Reports or Registries to identify All Patients Due for Cancer Screening for more details.
The care gap report should include the overall screening rate for colorectal cancer, breast cancer and cervical cancer and address the following:
- To apply an equity lens, screening rates should be disaggregated by:
- Race, ethnicity and language (REAL), sexual orientation and gender identity (SOGI) and similar data.
- Any known patient needs and preferences that would inform the screening modalities offered.
- Insurance status.
- Data on any other characteristics, including social needs, that could pose a barrier to completing screening.
3. Use data to determine the populations of focus for your reminders.
Suggested team member(s) responsible: Panel manager and multidisciplinary team for cancer screening.
- If your practice does not currently send reminders to patients for cancer screening, you may either elect to develop a broad reminder strategy in which every patient eligible and due for a cancer screening receives the same general reminder using the preferred communication method and/or tailored reminder strategy with customized messages and methods for one or more populations of focus (e.g. populations with low screening rates).
- If your practice currently sends any reminders to patients for colorectal cancer, breast cancer and/or cervical cancer screening, you can use data on the current effectiveness of this effort to decide whether you want to improve your broad reminder strategy for all patients eligible and due for screenings and/or develop or improve more tailored reminder strategy with customized messages and methods for one or more populations of focus.
- Most patients can benefit from reminders in their preferred language. We have provided links to general reminder materials in step four below.
- Start with frequent no-show or missed appointment patients and seek to understand their root causes, which may be related to health-related social needs or health literacy issues. Ask your care teams to identify patients they consider high-risk patients who have been lost to care.
- Some populations will require more customized culturally relevant reminders to increase their rates of recommended cancer screenings. We provide guidance and examples for developing reminders for specific populations of focus, in step five below.
4. Develop, refine and implement your practice’s general reminder strategy.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening, administrative staff, IT staff.
Substeps for implementing your general reminder strategy include:
- Determine the content for the initial reminder and any planned follow-up reminders. For the initial reminder, we strongly recommend incorporating patient-specific information, such as name, recommended screening and due date to personalize your practice’s reminders.
- Follow-up reminders should include additional content to explain the importance of getting screened now and may address the availability of support services to address barriers to screening. For example, common barriers to accepting colonoscopy include the time required to complete the test and transportation to a surgical center where the test is performed. A common barrier to cervical cancer screening is fear of the exam to procure the test. Subsequent messages may address such common barriers and offer assistance to overcome them.
- Strong sources of guidance and/or sample reminders for cancer screening include:
- California Department of Health Care Services: Every Woman Counts Reminder Cards for breast and cervical cancer.
- The Community Guide Cancer Screening: Client Reminders - Colorectal Cancer.
- The Community Guide Cancer Screening: Client Reminders - Breast Cancer.
- Tailor the method of delivery, the frequency of message delivery, and the message to subpopulations based on their access to and use of technology, language, ethnic background, and other sociocultural elements and based on patient and community feedback.
- Determine the communication method for reminders. This should include:
- Segmenting the list of patients to receive a reminder by the patient’s preferred communication method, if known. For patients for whom the preferred communication is not known, consider using an automated reminder based on the contact information you have for the patient.
- Using methods that your practice has the resources and bandwidth to sustain, including any automated reminders that your practice can provide.
- Potentially using one method for the initial reminder and different methods for follow-up reminders. Follow-up reminders may include more personal methods, such as phone calls from your care team.
- Schedule reminder delivery. This includes setting up automated reminders and follow-up reminders for patients who do not respond to initial reminders. Scheduling the reminders nearing a due date and follow-up reminders immediately following a due date may improve the effectiveness of the reminders. Depending on the bandwidth of your administrative team, this might include nonautomated phone calls as follow-up reminders to a subset of patients (e.g., patients at higher risk of cancer). Subsequent messages may need more tailored information that addresses common barriers to fulfillment of the recommendation, such as addressing transportation needs, addressing fears of screening, etc.
- Send out the reminders. Send out the reminders using the agreed upon content, communication method(s) and scheduled frequency. Studies have shown that women preferred receiving text messages about mammography on a regular basis.
- If the EHR allows, document the communication of the patient reminder, date and technique.
- Monitor delivery and responses. This includes:
- Monitor delivery rates, including bounce-backs and reminders flagged as undeliverable or similar. Attempt to find updated contact information for any reminder that couldn’t be delivered and update the EHR and/or contacts database with the new information.
- Track responses. Depending on the method(s) used, this might include tracking:
- Open rates (the reminder was opened).
- Responses (the patient responded to the reminder, regardless of outcome).
- Responses that result in initiating a cancer screening.
- Responses that result in completing a cancer screening.
- Track which methods yield better response rates for each of the above. Your practice can use this to improve (see step nine below).
5. Develop, refine and implement tailored reminders and outreach strategies for populations of focus.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening or their designees.
- Use your care gap reports and stratified data to identify populations or groups for whom your practice has lower screening rates and who might benefit from tailored strategies.
These populations vary but often include:- Patients of color.
- Patients for whom English is not their primary language.
- Immigrants and refugees.
- Patients with no insurance.
- Patients with behavioral health needs.
- Patients experiencing homelessness or housing instability.
- Patients with physical or developmental disabilities.
- Patients with lower literacy levels.
- Other historically marginalized populations.
- Determine which of the three populations you have the bandwidth to develop tailored strategies for, beginning with one population one, if needed):
- Patients who are regularly seen by the practice for other health-related reasons (active patients).
- Patients who have been seen by the practice in the past but not over the last 12 months (inactive patients).
- People who are on the practice’s panel but have not been seen by the practice (empaneled but not yet seen patients).
- Gather insights from the population of focus to better understand their needs and preferences regarding reminders and reminder methods. If you have existing messaging available, have members of the population of focus provide feedback on this including, what works, if anything; what was confusing, if anything; and what they think would need to be improved upon, if anything
- Use these insights to develop new ideas for reminders or to refine existing reminders for this population. Ideally, this ideation is done with representatives of the population of focus, as they have expertise and experience that may be missing from the practice’s care team. This might include having members of the population of focus:
- Develop the reminder message content. Having experts review to ensure compliance with clinical guidelines.
- Design or refine the layout for written messages.
- Send the message from a trusted member of their community rather than your practice.
- Change the timing of the reminder or follow-ups to the reminder.
- Change the reminder method or experiment with new methods.
- Using the insights and ideas you have developed, finalize the content for the reminders and implement the revisions. For the initial reminder, we strongly recommend incorporating patient-specific information, such as name, recommended screening, and due date to personalize your practice’s reminders. Follow-up reminders might include additional strategies to explain the importance of getting screened now.
- Use touches that provide alternatives to the traditional office visit. Assess what types of interaction or care can be provided outside this setting and collaborate with patients as part of initial care planning to understand their preferences for communication and care team interactions. Examples include:
- Nursing visits (virtual).
- Pharmacy visits.
- Digital communication through emails, texts and patient portals.
- Newsletters focused on a specific condition.
- Social media that includes discussion groups relative to the conditions of focus.
- Home visits for high-risk, historically marginalized or mobility-challenged patients.
- Other technological solutions, such as apps focused on patient conditions, which provide a vehicle for ongoing engagement.
6. Develop a follow-up system to proactively reach and engage patients who have not responded to reminders.
Suggested team member(s) responsible: Care team.
Segment your population. This action step should develop distinct strategies for three populations:
- Patients who are regularly seen by the practice for other health-related reasons (active patients).
- Patients who have been seen by the practice in the past but not over the last 12 months (inactive patients).
- People who are on the practice’s panel but have not been seen by the practice (empaneled but not yet seen patients).
Use the practice’s EHR or other tools to track patients who have not responded to reminders.
- Assign a member of the implementation team to track results of patient notification for cancer screening efforts and identify those who are eligible but have not responded within two months.
- Create a subregistry of those who have not yet responded to the recommendations.
- Determine if the patient is active (being seen by the practice), inactive (has been seen by the practice in the past but not in the past 12 months), or has never been seen (patient is on the panel but has never come into the practice).
Identify and attempt to correct errors in contact information.
- The designated care team member reviews the registry of those who have not responded and attempts to identify possible sources of error(s) (e.g., patient is no longer at the address, patient no longer has the phone number, etc.) and correct these errors.
- In cases where the practice is able to find updated contact information for a patient, the practice will resend the reminder to the patient.
Develop more focused outreach to patients for whom the practice appears to have correct contact information, but who have not responded to the general reminder(s). Since your practice’s bandwidth is limited, you might consider starting with your active patients, moving on to inactive patients once you are seeing success with your active patients, and tackling your not-yet-seen patients once you are seeing success with your inactive patients.
- For the practice’s active patients:
- The designated team member determines whether the patient has an upcoming appointment (within the next two months) at the practice for another reason.
- If so, the team member creates an alert in the patient record to notify the clinical team and provider about the patient’s eligibility for cancer screening (see Key Activity 9: Partner with Patients to Discuss Cancer Screening During Patient Visits).
- If the patient does not have an upcoming appointment, work with the care team to identify alternate methods to outreach to the patient, such as a community health worker (CHW) visit, cell phone text message, etc.
- If contact with the patient is achieved, discuss recommended screening with the patient, identify potential barriers, and work with the patient and team to offer strategies to address barriers (e.g., transportation or at-home sample procurement, such as fecal-based testing.)
- For the practice’s inactive patients:
- Start by determining if there are any other reasons to schedule a patient visit (e.g., annual visit, high blood pressure, diabetes, etc.).
- If there are other are other reasons for scheduling a visit, attempt to contact the patient to schedule the visit due to the reasons identified and, if they are willing to schedule a visit, create an alert in the patient record to notify the clinical team and provider about the patient’s eligibility for cancer screening (see Key Activity 9: Partner with Patients to Discuss Cancer Screening During Patient Visits).
- If there are no other obvious reasons for scheduling a visit, work with the care team to identify alternate methods to outreach to the patient, such as a CHW visit, cell phone text message, etc.
- If contact with the patient is achieved, discuss recommended screening with the patient, identify potential barriers, and work with the patient and team to offer strategies to address barriers (e.g., transportation or at-home sample procurement, such as fecal-based testing.)
- For people who are on the practice’s panel but have not been seen by the practice (empaneled, but not yet seen patients):
- Work with the care team to develop a customized message for the person to consider coming into the practice for care, and identify alternate methods to outreach to the patient, such as a CHW visit, cell phone text message, etc.
- If contact with the patient is achieved, discuss recommended screening with the patient, identify potential barriers, and work with the patient and team to offer strategies to address barriers (e.g., transportation or at-home sample procurement, such as fecal-based testing.)
For going deeper in this area, consider Key Activity 15: Strengthening Community Partnerships to provide outreach to others outside of your patient population. Community partners can support outreach and health literacy efforts, participate in collaborative design by providing insight around a particular patient population, and provide resources and support for patients. See also Key Activity 10: Use Culturally Appropriate Educational Materials for Cancer Screening.
7. Track the effectiveness of your reminder and outreach strategy.
Suggested team member(s) responsible: Quality improvement lead or their designee.
For the overall population of patients due for cancer screening, as well as the subpopulations your practice is focusing on, regularly (at least quarterly) use the data from your care gap reports to determine if your strategies are increasing the percentage of patients who are having all recommended screenings while reducing inequities in screening rates.
8. Put in place formal and informal feedback loops with patients and the care team.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening or their designees.
To help ensure that educational materials meet the needs of patients and the processes involved are consistently feasible for the care team, it is important to have both formal and informal feedback loops.
- For patients, feedback loops might include:
- Sharing current reminders to gather feedback and incorporate that feedback into revised reminders.
- Getting verbal feedback from patients directly after testing a new reminder or a new reminder strategy with them and incorporating their feedback into your next test.
- Patient satisfaction surveys (or similar).
- Follow-up calls with a subset of patients to understand what works well and what could be improved.
- Patient focus groups.
- Having the practice’s patient advisory board (or similar body) provide feedback.
- For the care team, feedback loops might include:
- Daily huddles.
- Existing or new staff satisfaction and feedback mechanisms.
- Regularly scheduled meetings and calls to get staff feedback on the cancer screening reminder systems.
9. Use quantitative and qualitative data (see steps six and seven above) to improve the effectiveness of reminders.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening or their designees.
This ongoing work might include:
- Refining the content of reminders, based upon feedback.
- Modifying the communication method(s) used for reminders.
- Further automating reminders.
- Developing tailored reminder strategies for one or more populations of focus who have lower reminder response rates.
- Using human-centered design methods to develop new ideas.
- Using quality improvement strategies, methods and tools to improve areas of focus.
10. Cultivate a trusting and engaging environment where patients feel comfortable in accessing care.
Suggested team member(s) responsible: Care team.
- Collaboratively designed outreach and engagement strategies with community partners and people using the practice’s services.
- Train all staff in cultural humility, motivational interviewing skills, and the use of trauma-informed care.
- Sponsor peer groups and group visits of similar patient segments of your population to foster peer engagement and support.
- Sponsor support groups for caregivers of patients with chronic conditions.
- Leverage your current patients and staff as potential trusted messengers to engage patient subgroups.
This resource provides recommendations and considerations to ensure comprehensive care for when patients decline colorectal cancer screening. Access the full resource.
Implementation tips
- Where there is a backlog of patients due to address a gap in care, consider a campaign where you bring patients in who are overdue for similar screenings, labs or vaccines.
- Consider scheduling outreach to these patients on days when you might experience a lower volume of activity in order to level the demand on the care team.
- Utilize multiple pathways to reach and engage patients.
- Communicate where potential patients are most comfortable.
- Regularly update outreach strategies based on community feedback and changing demographics.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
Evidence base for this activity
Denberg TD, Myers BA, Eckel RH, mcdermott MT, W. Perry Dickinson, Lin CT. A patient outreach program between visits improves diabetes care: a pilot study. International Journal for Quality in Health Care. 2009 Apr 1;21(2):130–6.
Ntiri SO, Swanson M, Klyushnenkova EN. Text Messaging as a Communication Modality to Promote Screening Mammography in Low-income African American Women. Journal of Medical Systems. 2022 Apr 13;46(5).
Subramanian S, Tangka FKL, Hoover S, DeGroff A. Integrated interventions and supporting activities to increase uptake of multiple cancer screenings: conceptual framework, determinants of implementation success, measurement challenges, and research priorities. Implementation Science Communications. 2022 Oct 5;3(1).
KEY ACTIVITY #7:
Create and Use Clinician Reminders
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; proactive patient outreach and engagement; pre-visit planning and care gap reduction.
Overview
This activity provides guidance on how to develop, configure and use reminders in the electronic health record (EHR) so that clinicians are aware of which patients are eligible for and due for their colorectal cancer, breast cancer and/or cervical cancer screening based on the U.S. Preventive Services Task Force (USPSTF) guidelines. This activity calls for clinician reminders that flag patients at higher risk, as well as patients with likely barriers to screening (e.g., mobility issues, speaking a language other than English, etc.).
While many of the examples for this activity are for colorectal cancer screening, in most cases the same general steps apply to other adult cancer screenings. When the steps need to be customized for breast cancer or cervical cancer screening, we have included specific guidance.
Clinician reminders are one of several tools that members of the care team can use to help ensure they are aware of their patients’ need for colorectal, breast and/or cervical cancer screening and can discuss screening as part of patient encounters or outreach efforts. These reminders can reduce the time needed to identify the preventive services recommended for each patient.
This activity relies on similar capabilities as care gap management, utilizing population views and registries to create patient specific reminders within each patient’s chart. For EHRs that have the capacity to display care gaps, that information can be leveraged to create clinician reminders for patients who might have an upcoming appointment. While that technology is not well developed, information from registries and care gap reports can be utilized to generate lists that enable care team members or other clinical staff the opportunity to create those reminders. Many EHRs are capable of storing next-appointment data that can also be used to differentiate patients who need a clinical reminder versus those who would benefit from outreach.
Other relevant HIT capabilities to support this activity include care guidelines, clinical decision-making support, including patient-facing clinical decision support, and care dashboards and reports.
Action steps and roles
1. Create a flag or alert in the patient record.
Suggested team member(s) responsible: Panel manager or data Analyst and practice staff responsible for the EHR.
At the patient level, ensure that the practice’s electronic health record (EHR) or population health management tool creates a flag or alert to notify the care team of gaps in care related to colorectal cancer, breast cancer and cervical cancer screening using the latest USPSTF recommendations for colorectal cancer and USPSTF or other recommendations for breast cancer and cervical cancer, in accordance with your practice’s cancer screening protocols (see Key Activity 2: Develop or Update the Practice’s Cancer Screening Protocols). These alerts enable the care team to maximize the identification of necessary preventive services related to patient visits.
See also the clinical practice guidelines section earlier in this guide.
This step may involve one or more people from the practice who work on the EHR, if it is not clear if or how the EHR can produce the colorectal cancer, breast cancer and cervical cancer screening care gap reports.
An example of what a patient-level care gap alert may look like is included in Figure 13.
FIGURE 13: EXAMPLE POPULATION CARE GAP REPORT ON BREAST, CERVICAL, AND COLORECTAL CANCER SCREENING
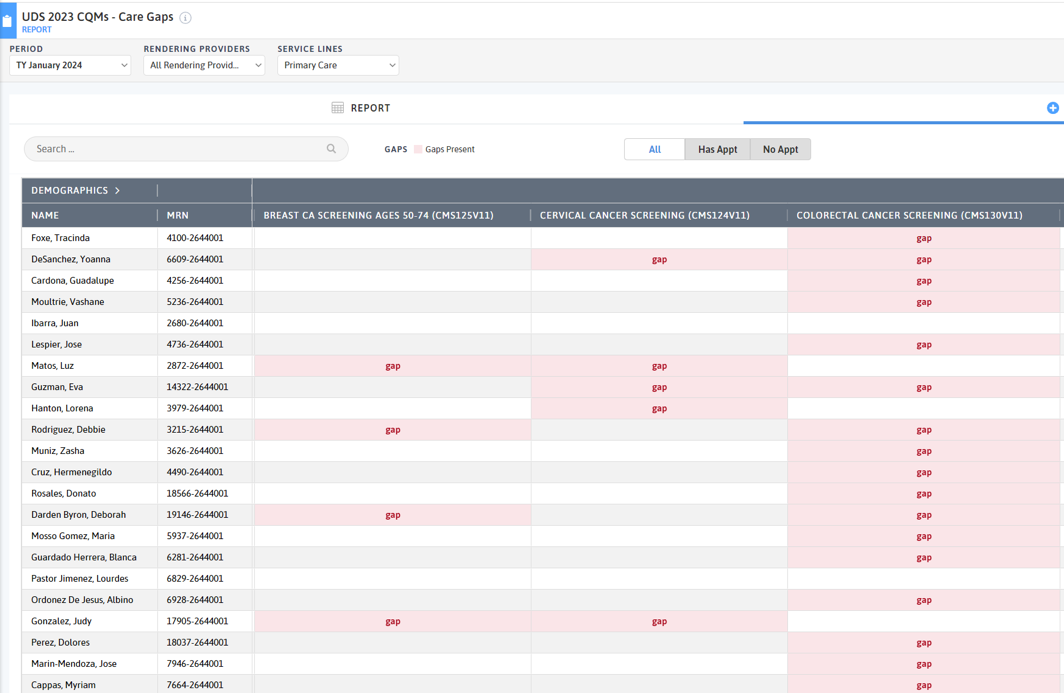
2. Configure the EHR alerts.
Suggested team member(s) responsible: Practice staff responsible for the EHR.
Collaborate with the person or team responsible for your EHR to set up automated alerts within the EHR system for patients due for colorectal cancer, breast cancer and/or cervical cancer screening.
Define reminder frequency. Determine the timing and frequency of reminders (e.g., annually, biennially) based on clinical guidelines and patient risk factors.
Work with the care team and the person or team responsible for your EHR to help ensure that the automated alerts are not lost due to the flood of alerts they receive, are provided at the appropriate time, and are in a place or on a screen that is intuitive to the care team.
If possible, the alert also flags those at greater risk (see the USPSTF guidelines) and/or those who likely have barriers to getting screened.
3. Make clinician reminders a part of the pre-visit planning process.
Suggested team member(s) responsible: Panel manager.
Work with the clinician and care team to develop ways to ensure these reminders are part of the pre-visit planning process. See Key Activity 8: Refine and Implement a Pre-Visit Planning Process.
4. Ensure reminders are updated continuously.
Suggested team member(s) responsible: Panel manager, QI lead and/or their designee.
So that the clinician reminders remain accurate (e.g., don’t show a patient needing a cancer screening that they just had), it is important to ensure a process for updating the EHR records. At a minimum, this should include:
- The person(s) responsible for doing this.
- Whether the needed screening was discussed with the patient (yes/no).
- If the screening was discussed and the patient’s response (e.g., yes, maybe, no, not at this time).
- The follow-up needed based on this conversation, including the results of any screening.
5. Provide ongoing training on creating and using clinician reminders.
Suggested team member(s) responsible: Panel manager and practice staff responsible for the EHR.
Ensure that all members of the care team receive ongoing training on each of the four steps above. This training can be incorporated into huddles and should be part of the orientation for new staff. At a minimum, it should include ensuring that care team members:
- Know of the reminders and what they mean.
- Understand where, how and when they will receive them. This should include showing each clinician in the EHR itself and ensuring that this flag or alert is turned on, as well as visual reminders easily accessible to clinicians.
- Understand the process for updating the EHR to ensure that the reminders are accurate.
6. Monitor clinician reminders for accuracy and completeness.
Suggested team member(s) responsible: Panel manager, QI lead and/or their designee.
It is critical to have a feedback loop with the practice’s care team about any real or potential errors in clinician reminders. This might include patients who are eligible for and due for screening for whom a reminder or flag does not appear or patients who have recently been screened for whom the reminder or flag continues to appear. The reminders process should be modified as needed whenever errors are discovered.
FIGURE 14: EXAMPLE OF A CLINICIAN REMINDER
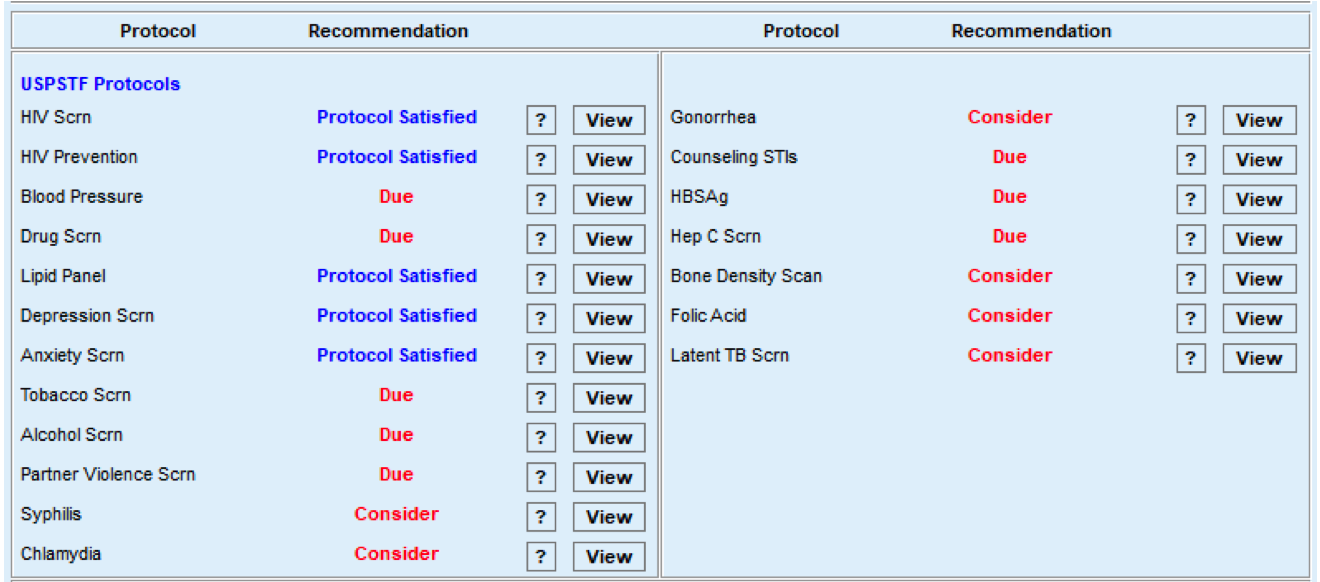
Implementation tips
- If the practice has successfully implemented clinician reminders in other clinical areas, this can be used as the basis for clinician reminders for cancer screenings.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
KEY ACTIVITY #8:
Refine and Implement a Pre-Visit Planning Process
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; pre-visit planning and care gap reduction; care coordination; address social needs.
Overview
This activity provides guidance for how the care team can effectively and efficiently embed cancer screenings into the practice’s pre-visit planning process (PVP). Pre-visit planning is typically driven by the medical assistant with help from other care team members and includes steps taken:
- At the end of the current visit to ensure the patient understands any actions they need to take and to schedule any follow-up.
- Prior to a scheduled appointment, scrub the chart and identify any pre-visit tasks per the pre-visit checklist.
- The day of an appointment, during the daily huddle and before the patient sees the PCP.
The average visit time for U.S. primary care providers is 13 to 24 minutes, [13]and many patients come to these visits with multiple needs. Pre-visit planning works towards optimizing a team-based approach in preparation for these short primary care visits so patients receive comprehensive care in alignment with the latest clinical guidelines and their own preferences.
Pre-visit planning allows for better coordination of care and works to maximize opportunities to address multiple health-related needs. This can be particularly beneficial for patients with complex health needs, ensuring they receive comprehensive and equitable care. As your practice works to reduce any identified equity gaps, pre-visit planning is often a powerful activity for ensuring culturally relevant care as the care team partners with the patient to discuss follow-up actions.
The PVP should include information regarding each patient’s known social needs and incorporate your practice’s process for screening and responding to social needs. Social needs may be screened by an MA and any outstanding questions reviewed with the team or social worker in the daily huddle. The PVP should incorporate your practice’s process for screening and responding to social needs, including checking whether social needs screening is due. When social needs are identified, the team should be clear on the pathways, both during and after visits, to address and follow up on those needs.
Pre-visit planning draws upon similar technical enablers as care gap reporting and likewise can be facilitated at the individual patient level and at the level of groups of patients coming for care by a specific team in an appointment schedule block. The format in which planning is done needs to consider the workflow and staffing model.
Relevant HIT capabilities to support this activity include care guidelines, registries, clinical decision support, care dashboards and reports, outreach and engagement, and care management and care coordination (See Appendix E: Guidance on Technological Interventions.)
Individual patient-level pre-visit planning would optimally be enabled within the EHR, provided the EHR is able to store relevant information, such as assessments, plans, orders and notes outside of a visit note. Pre-visit planning might also include use of patient-facing applications, such as portal reminders, questionnaires, and self-completed screenings and assessments, which ideally would be available to the provider and care team in the EHR.
Engagement of the expanded care team in pre-visit planning requires access by all relevant members of the care team to contribute to and view relevant information. This may require coordination with technology additional to the EHR, such as care coordination and population management applications where relevant information might be stored.
Huddle reports, in which individual patient information can be visualized in summary views across all patients within a session, can require use of technology outside the EHR, but might also be supported by developing reports populated by data from the EHR. Ideally, internal practice and EHR data should be supplemented with external data where such information completes the patient’s current status. If such electronic access is not possible, the workflow should include manual reconciliation by history from the patient.
Figure 15: An example of how technology can help with pre-visit planning.
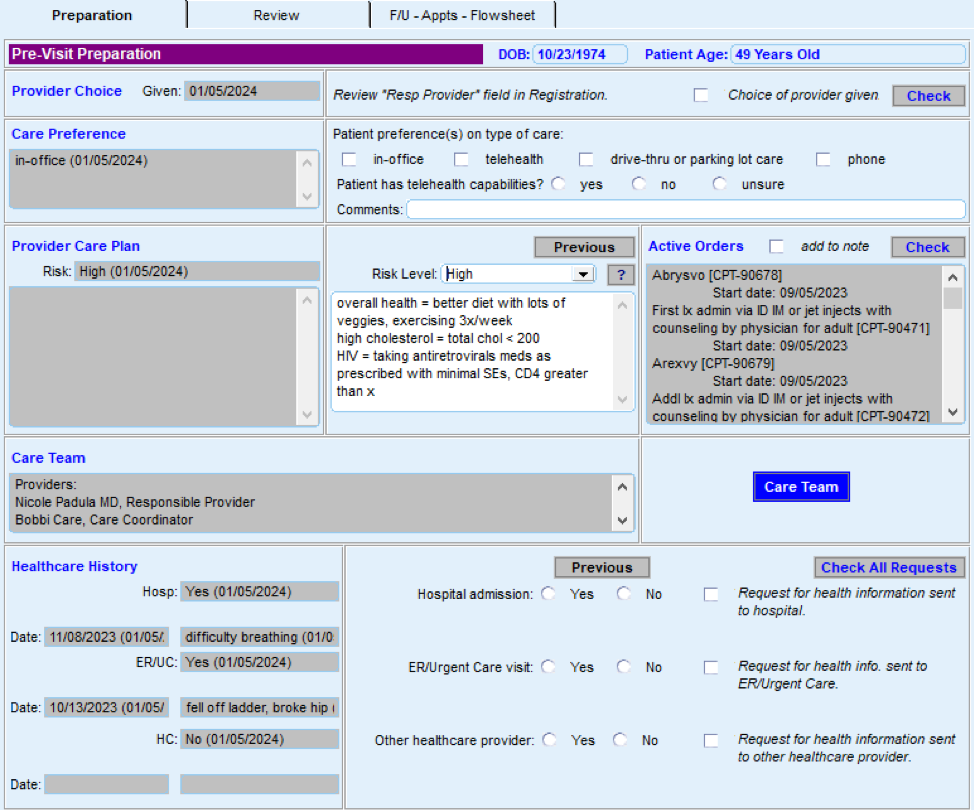
Action steps and roles
1. Assess the current state of the pre-visit planning (PVP) process across your practice.
Suggested team member(s) responsible: Implementation team or QI team.
- This step begins by working to understand how the process currently works based on your practice’s performance on core and supplemental measures.
- Compile existing paperwork and process documentation into one place and talk to medical assistants and other members of the care team to understand their questions and challenges around the pre-visit planning process.
- Consider making current-state process maps that communicate the major steps in the PVP process and acknowledge pain points or kinks in the process. Pain points are places where the process isn’t working well for patients, staff or specific subpopulations or demographic groups for which your data analysis has revealed equity gaps. In addition, pay attention to places where staff are creating workarounds to the process. The presence of workarounds indicates that the process might need further clarification or refinement to support the care team to reliably implement it.
- Your team may find it helpful to compile process maps for both the high-level pre-visit planning, as detailed in figure 6.1 of the PHMI Care Teams and Workforce Guide Resource 6: Workflow Examples, in addition to more detailed documentation for specific clinical workflows. Understand which clinical workflows and care gaps are currently addressed through the PVP process and work to understand what gets in the way of the team reliably addressing these care gaps.
FIGURE 16: EXAMPLE WORKFLOW FOR PRE-VISIT PLANNING (PVP).
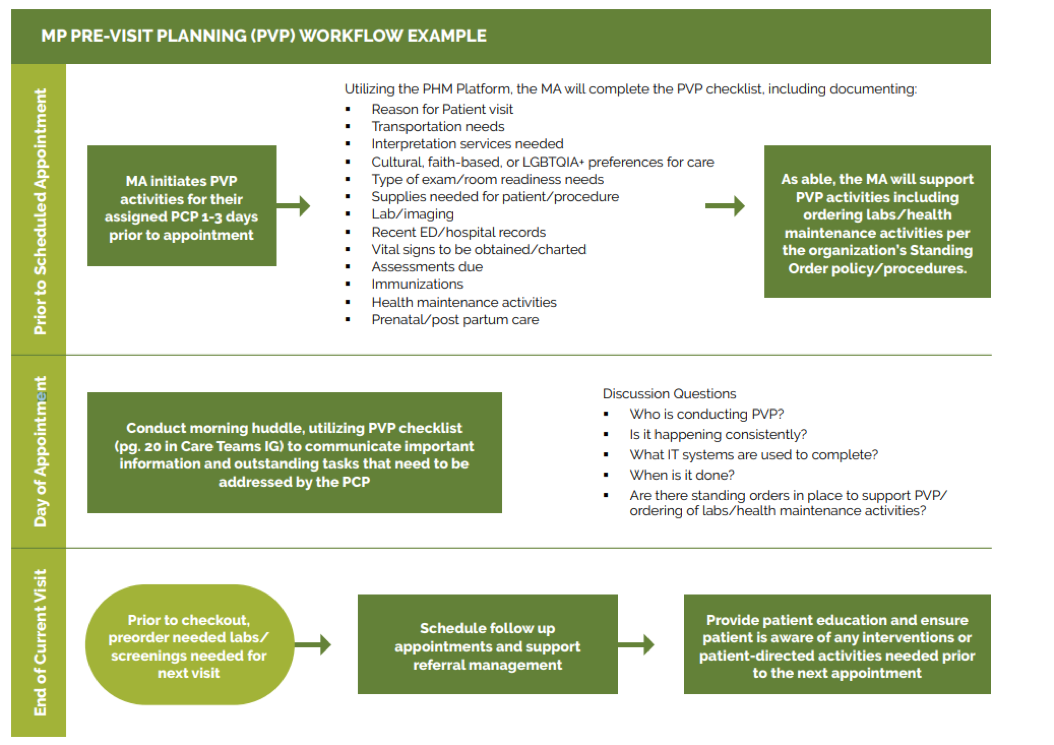
2. Identify where to update the PVP process to address care gaps more consistently and reliably.
Suggested team member(s) responsible: Implementation team or QI team; may include the data manager to assist with identifying patients through EHRs.
Examine where you can further incorporate or streamline your practice’s clinical protocols and standing orders into PVP, including via the PVP checklist, daily huddles and other key clinical workflows.
This may include the following discussion points:
- Who will initiate the discussion with the patient based on the needs (e.g., primary language) and preferences of the patient.
- How and when they will plan to have the discussion during the visit.
- How they will be prepared to address any barriers.
- Educational materials available for use that are relevant to the patient.
Clinical guidelines and care gaps: Consider which preventive and maintenance services could be addressed more reliably using PVP and include those in your EHR’s care gap module. The Pre-Visit Planning: Leveraging the Team to Identify and Address Gaps in Care resource provides a more complete list of preventive services for adults and children.
PVP checklist: A generic PVP checklist is to be completed by the MA or LVN at least one to three days before the appointment is provided in Figure 17. Most EHRs will have a health maintenance module that identifies what preventive care is due, but a printed or digital checklist provides a starting point for ensuring key steps aren’t missed.
FIGURE 17: PVP PLANNING CHECKLIST ITEMS TO ADD FOR ADULT PREVENTION
|
Checklist Domain |
Checklist Item |
|---|---|
General |
|
Room readiness |
|
Medical record review |
|
Screenings are completed by the patient, results are documented, and follow-up is completed. |
For a more complete list of preventive screenings, see the resource Pre-Visit Planning: Leveraging the Team to Identify and Address Gaps in Care.
|
Scrub chart for care gaps, preclinic labs, and missing information: This is not an all-encompassing list of what should be included for PVP, but instead serves as a starting point to address core HEDIS measures. |
An example chart scrub process led by the MA or LVN as part of PVP for completing open orders is detailed in Figure 18.
|
FIGURE 18: EXAMPLE CHART SCRUB PROCESS FOR OPEN ORDERS.
Putting it all together: Leveraging team-based approaches to address screening for cervical, colorectal and breast cancer
Pre-visit planning checklists may be created by the multidisciplinary team, authorized by the medical team, and carried out by relevant health center staff. The list below provides examples of possible actions that care team members can take to support providers and to advance population health management for both in-person visits or outreach attempts to identify and address gaps for cervical, colorectal and breast cancer screening.
For breast, cervical and colorectal cancer, MAs or nurses (or others on the team) can:
- Advise the patient they are due for the screening.
- Provide patient education on the benefits of completing the screening.
- Outline screening options for the patient.
- Enter orders into the EHR in accordance with standing orders (see Key Activity 5: Develop and Implement Standing Orders).
- Assist or refer patient to ensure insurance or other program coverage and to advise of any potential out-of-pocket costs.
- Schedule patient, if possible. If not, provide information to the patient to schedule and set a reminder to call patient to ensure they were able to get scheduled.
- If a patient declines, document accordingly, including why the patient declines, and notify the provider to have further discussions and to document that the patient declines after informed consent.
Daily huddles: Design or redesign the team’s daily huddle so the care team can review prioritized action items for the list of patients scheduled to come and any further follow-up for patients seen in the previous days. For more information, see PHMI Care Teams and Workforce Guide Resource 4: Daily Huddles Overview and Process.
The Centers for Excellence in Primary Care resource on Healthy Huddles includes tools, a video, and examples around how pre-visit planning flows into the daily huddle process, including a “Healthy Huddles” warm-up, which can help MAs prepare for huddles.
Patients requiring special considerations: Ensure that the PVP includes a process to flag for the care team:
- Patients requiring special attention, such as those with chronic conditions, complex needs or recent hospitalizations.
- Patients who need extended time or additional services during their visit (e.g., translation services, mobility assistance, screenings).
Detailed clinical workflows: Clinical workflows should be updated to describe at which step in PVP or the patient visit a care team member will address a specific gap in care with the patient.
Planning forward for care after the visit: Effective PVP includes scheduling pre-visit labs and other diagnostics at the end of the current visit and coordinating care and referrals for services not available at the practice (see Key Activity 13: Coordinate Care). Confirm the patient understands the purpose of any follow-up care.
The American Medical Association (AMA) has an online guide for implementing pre-visit planning, which includes forms, templates and other resources.
3. Test planned changes to the PVP workflows and support care teams in implementing the workflow.
Suggested team member(s) responsible: Implementation team or QI team.
Test and refine important changes to the PVP process with one or two care teams before rolling out the change more broadly at the practice.
Implementing the change includes updating documentation and creating a staff training schedule or training refresher for staff to sustain workflow changes. For example, train MAs every six months on the PVP. If they have difficulty, have them retake training with a different highly effective MA and monitor closely until competency in the task is reached.
4. Establish a process to review and update PVP workflow.
Suggested team member(s) responsible: Care team with quality improvement manager.
Identify staff responsible for reviewing and updating the PVP process at least annually to incorporate the latest clinical guidelines and check for any workarounds that have developed.
Implementation tips
- If the previous visit noted the need for laboratory testing for the next visit, the assigned team member should check to see if the testing has been completed. If not, the patient should be contacted to discuss this with the patient and recommend testing prior to the visit. This maximizes the value of the visit.
- Pre-visit questionnaires, sent electronically or by mail in advance of the visit or completed in the waiting room, can provide additional information that the care team can review prior to the patient’s visit.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
KEY ACTIVITY #9:
Partner with Patients to Discuss Cancer Screening During Patient Visits
This key activity involves the following elements of person-centered population-based care: : operationalize clinical guidelines; proactive patient outreach and engagement; care coordination; address social needs.
Overview
This activity provides guidance on how the practice’s care team will use patient visits scheduled for other reasons as an opportunity to discuss any needed colorectal, breast and/or cervical cancer screening and provide education, including choice of testing, where feasible, to patients.
Numerous studies[14] [15] have demonstrated that having a clinician discuss the need for cancer screening directly with a patient is among the most effective and efficient strategies to support fulfillment of screening recommendations for colorectal, breast and/or cervical cancer. Developing skills to enable these critical conversations will increase the likelihood of success. In addition, patient education, discussing the benefits and risks of cancer screening, and offering a choice of testing modalities, where feasible, have all demonstrated improvement in cancer screening rates.[16] Discussions about cancer screening should also address concerns the patient has about engaging in screening activities so that these can be taken into consideration and addressed.
The approach to this discussion cannot be one-size-fits-all and needs to be tailored to the needs, preferences, culture, and likely barriers to screening of each patient. This activity provides guidance for better tailoring the conversation for several historically vulnerable populations. This should include the care team asking the patient about their concerns regarding the screening and about barriers or concerns in accessing the screening, many of which are related to social needs. Clinicians should explain the risks and benefits of the test and have relevant educational materials available.
It is important that data fields and workflows are configured to accurately capture preventive care declination to understand and manage this common challenge. Patient outreach and engagement technologies can assist the care team in assessing hesitancy, as well as delivering education. These technologies can be programmed to deliver scripted responsive interactions that can extend the ability of the care team to spend time addressing concerns and questions. Care dashboards and quality reports can be utilized with data analytics to identify trends that might suggest population-level strategies. Additionally, care should be exercised to understand population differences that might be contributing to patient declination. The use of data stratified by health-related social needs and other equity markers is encouraged to identify barriers to screening. Clinical templates and/or prompts in the EHR and care coordination applications can guide provider and care team discussions.
As outlined elsewhere, use of patient-facing technology can be utilized to augment direct discussion through education. Increasingly, these applications can be utilized in interactive ways and may be implemented in ways to provide options to engage the care team.
Action steps and roles
1. Use pre-visit planning and/or huddles to initiate this process.
Suggested team member(s) responsible: Primary care provider (PCP) and medical assistant (MA) for each panel.
This process starts during daily pre-visit planning and huddles by identifying which patients will need a reminder about the need for cancer screening(s) and the plan for having this conversation during the patient visit, including who will initiate the conversation and when. Patients who are eligible for screening tests should be flagged so that the PCP recognizes the need for guidance and recommendation. For more information, see Key Activity 8: Refine and Implement a Pre-Visit Planning Process and PHMI Care Teams and Workforce Guide Resource 4: Daily Huddles Overview and Process.
2. During the patient visit, discuss the need for colorectal, breast and/or cervical cancer screening and the importance of the screening(s) with the patient.
Suggested team member(s) responsible: Designated care team member, based on pre-visit planning.
The designated care team member should initiate the conversation about the need for screening. Based on the patient's age, risk factors, and medical history, the care team member should explain the importance of cancer screening. Effective communication strategies include:
- Asking open-ended questions about the patient's health, well-being and health goals.
- Listening attentively to patient questions or reservations and providing accurate, empathic answers.
- Promoting shared decision-making with the patient.
This should be done in the preferred language of the patient, using interpretation services as needed. Use or adapt existing curricula, scripts, patient materials, visuals and/or videos to help patients understand the importance of cancer screening. See the resources for this activity below.
Preventive healthcare includes participation in select screening tests that have been shown to identify disease early—before symptoms develop—and when treatment is most likely to be effective. This guide addresses steps to support screening for patients at average risk for breast, cervical, and colorectal cancer. Those who are at average risk should be encouraged and supported to undergo screening tests at the frequency noted in the PHMI clinical guidelines.
Some people, however, are at elevated risk for certain types of cancer, and they should be screened using specific screening methodologies at intervals that may differ from routine screening. Those at elevated risk should not be advised to follow the routine screening guidance outlined in the Implementation Guides but should follow individualized screening guidance in accordance with their risk for a particular disease.
Conditions and circumstances that elevate a person’s risk may be related to the presence of signs or symptoms suggestive of cancer, a personal history of cancer or of pre-cancerous conditions, a close family member with a specific cancer or certain types of exposure. When determining if a person is at elevated risk, it is useful to review their health history and ask the following questions at regular intervals, because an individual’s risk status may change over time:
- Have you ever been diagnosed with breast/cervical/colorectal cancer or a precancerous condition?
- Do you have a first degree relative (biological parent or sibling) who has been diagnosed with cancer or a precancerous condition?
- Do you have a genetic marker or syndrome associated with higher cancer risk?
- Do you have any medical conditions that place you at higher risk for cancer (for example, Crohn’s disease increases the risk for CRC cancer)?
- Have you had exposure to drugs or radiation that might affect your risk for cancer?
If a patient is identified as having an elevated risk for breast, cervical, or colorectal cancer, the primary care provider should discuss this risk with the patient and advise the patient of the modified recommendations for persons at elevated risk.
For additional information concerning increased risk for breast cancer, review the following guidance:
- American Cancer Society Recommendations for the Early Detection of Breast Cancer
- US Preventive Services Task Force Full Recommendation for Breast Cancer Screening, Practice Considerations
For additional information concerning increased risk for cervical cancer, review the following guidance:
- US Preventive Services Task Force Cervical Cancer: Screening Recommendations, Clinical Considerations
- Cervical Cancer Screening for individuals at average risk: 2020 guideline update from the American Cancer Society
For additional information concerning increased risk for colorectal cancer, review the following guidance:
- US Preventive Services Task Force Recommendations for Colorectal Cancer: Screening, Practice Considerations
- American Cancer Society Guideline for Colorectal Cancer Screening
Guidance Provided by:
- Margaret Franckhauser, MS, MPH, RN
- Adults with Preventive Health Needs Subject Matter Expert
References:
- U.S. Preventive Services Task Force. U.S. Preventive Services Task Force [Internet]. www.uspreventiveservicestaskforce.org. U.S. Preventive Services Task Force; 2022 [cited 2024 Dec 9]. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/
- American Cancer Society. Cancer Screening Guidelines | Detecting Cancer Early [Internet]. www.cancer.org. American Cancer Society; 2023 [cited 2024 Dec 9]. Available from: https://www.cancer.org/cancer/screening/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html
3. As needed, discuss the benefits and potential risks of colorectal cancer, breast cancer and/or cervical cancer screening with the patient.
Suggested team member(s) responsible: Designated care team member, based on pre-visit planning.
Use or adapt existing curricula, scripts, patient materials, visuals and/or videos to help patients understand the benefits and potential risks of cancer screening. This can include any differences in benefits or risks for the choice of screening modalities appropriate for them.
See the resources for this activity section below.
4. Discuss the choice of screening modalities with the patient when this is an option, and allow patients to select their preferred method.
Suggested team member(s) responsible: Designated care team member, based on pre-visit planning.
Offering patients a choice in healthcare screening methods, where choices exist, can increase the rate of screening completion. For example, patients are less likely to undergo colorectal cancer screening if the primary care practitioner only recommends colonoscopy, but screening rates increase when the provider offers the option of at-home fecal testing. Offering patients a choice allows them to address barriers to care, such as transportation, time limitations, or fear of anesthesia. Offering options places the patient in a position of authority over their own care.
See the resources for this activity section below
5. Discuss which screening is covered and any out-of-pocket costs for cancer screening with the patient.
Suggested team member(s) responsible: Designated care team member, based on pre-visit planning.
Helping ensure that the recommended screening(s) are covered or low-cost may involve working with the patient’s health insurer, if one exists, modifying the timing of the screening, and other strategies. For patients who are not on Medi-Cal, this may require exploration with the insurer to verify coverage. Use or adapt existing curricula, scripts, patient materials, visuals and/or videos to help patients understand what is covered and what the out-of-pocket costs are related to colorectal cancer screening. See the resources for this activity section below.
6. As needed, discuss barriers to screening with the patient.
Suggested team member(s) responsible: Designated care team member, based on pre-visit planning.
Many patients have barriers to screening. To help reduce barriers (e.g., transportation, mobility, instructions in their preferred language, etc.), have an open discussion with each patient to understand the barrier(s) and co-develop potential ways to address each barrier with the patient. After the discussion, document the barriers and any strategies to address them in the EHR.
The Community Guide, endorsed by the American Academy of Family Physicians (AAFP), discusses specific interventions that can address structural barriers to breast, colon and cervical cancer screening.
7. Use quality improvement (QI) methods to test and refine all changes before bringing them to full scale at the practice.
Suggested team member(s) responsible: Panel manager and QI lead or their designee.
Whenever trying a new or adapted script, educational resource, or process, use plan-do-study-act (PDSA) cycles. We recommend starting with small-scale tests (e.g., test with one patient or for one afternoon). Study the results of the test and then refine the process, script or educational resource, as needed, as you test again. Continue increasing the scale of your tests as you see them achieving better results under a wider range of conditions.
While this may appear like it would slow down the full implementation of the change, by working out the inevitable kinks in the process before taking it to full scale, the practice will actually save time, resources, and frustration, while making the process better for patients. Testing and refining also can eliminate the costly workarounds that occur when a policy or system doesn’t fit well into the system or workflow it is being placed into. Generally, start as small as feasible – think “ones” – (e.g., one clinician, one hour, one patient, etc.) and become larger as your degree of belief in the intervention grows.
Selected resource on quality improvement (QI):
- Institute for Healthcare Improvement (IHI) QI Essentials Toolkit.
- IHI’s Videos on the Model for Improvement (Part one and Part two).
- Rapid Experiments to Improve Healthcare Delivery.
- CCI’s ABC's of QI via CCI Academy.
8. Put in place formal and informal feedback loops with the care Team and patients.
Suggested team member(s) responsible: Panel manager and QI lead or their designee.
To help ensure this activity is meeting the needs of patients and is consistently feasible for the care team, it is important to have both formal and informal feedback loops.
For patients, feedback loops might include:
- Patient satisfaction surveys (or similar).
- Follow-up calls with a subset of patients to understand what went well and what could be improved.
- Patient focus groups.
- Having the practice’s patient advisory board (or similar) provide feedback.
For the care team, feedback loops might include:
- Existing or new staff satisfaction and feedback mechanisms.
- Regularly scheduled meetings and calls to get staff feedback on processes, methods and tools.
See also the Key Activity 10: Use Culturally Appropriate Educational Materials for Cancer Screening and Key Activity 11: Provide or Arrange for Cancer Screening for guidance on providing screening to patients who express interest in this.
Implementation tips
- Always try to test each action or substep on a very small scale to work out kinks and challenges before scaling up the action or substep. This will save time and frustration.
- Consider how your existing technology can be leveraged to automate or partially automate the steps in this activity (e.g., EHR, automated survey mailers, email platforms, etc.).
- Combining clinician counseling and patient education materials improves patient likelihood of follow-through.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity
- See also Key Activity 3: Use Care Gap Reports or Registries to Identify All Patients Due for Cancer Screening for guidance on differentiating between average and elevated risk for cancer screening.
Resources
Evidence base for this activity
Alberti LR, Garcia DP, Coelho DL, De Lima DC, Petroianu A. How to improve colon cancer screening rates. World J Gastrointest Oncol. 2015 Dec 15;7(12):484-91.
Dalton AF, Golin CE, Morris C, et al. Effect of a Patient Decision Aid on Preferences for Colorectal Cancer Screening Among Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2022;5(12):e2244982. doi:10.1001/jamanetworkopen.2022.44982.
KEY ACTIVITY #10:
Use Culturally Appropriate Educational Materials for Cancer Screening
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; proactive patient; care coordination; address social needs.
Overview
This activity provides guidance on how practices can curate and use existing educational materials to increase the rate of patients who complete recommended colorectal cancer, breast cancer and cervical cancer screening.
Numerous studies[17][18][19][20][21][22][23] have demonstrated that patient education – both self-directed and guided by a member of the care team – is an effective and efficient strategy for getting patients screened for colorectal, breast and/or cervical cancer. Patient education about their risk of cancer, the recommended screening tests, the risks and benefits of the testing methods, and how the tests are performed help to support informed decision-making. Education is more likely to be followed, if it is delivered in ways that are meaningful to the patient, culturally relevant and linguistically appropriate.
See also Key Activity 20: Develop Customized, Culturally Appropriate Educational Materials for guidance on how to develop new materials for one or more subpopulations.
Many EHR platforms allow for the development or customization of embedded patient education material. Those tools can then be available for all care team members to share with patients in one of several modalities: at the health center, by mail, or via patient outreach and engagement technologies. Patient outreach and engagement technologies can assist the care team in assessing screening hesitancy, as well as delivering education. These technologies can be programmed to deliver scripted responsive interactions that can extend the ability of the care team to spend time addressing concerns and questions. Care dashboards and quality reports can be utilized with data analytics to identify trends that might suggest population-level strategies. Clinical templates and/or prompts in the EHR and care coordination applications can guide provider and care team discussions.
Action steps and roles
1. Understand the patient population(s) at your practice eligible for screening.
The first step is to understand the patient population eligible for colorectal cancer, breast cancer and cervical cancer screening at your practice.
Suggested team member(s) responsible: Panel manager or data analyst and multidisciplinary team for cancer screening.
This information can be found in your practice’s care gap reports for colorectal cancer screening, breast cancer screening and cervical cancer screening. See Key Activity 6: Conduct Proactive Outreach to Patients Due for Screening for more details.
2. Compile, curate, evaluate and adapt existing educational materials.
Suggested team member(s) responsible: Multidisciplinary team, MA, nurses, health educators.
Based on your patient population, compile, curate, evaluate and adapt your practice’s existing educational material for colorectal cancer, breast cancer and cervical cancer screening.
Assess the materials to determine if they address population needs and are understandable by the patient populations for your practice. Review reliable sources for educational materials that have been developed and tested by reputable sources.
See the Resources section of this Activity for reputable sources of educational materials.
It may be useful to categorize the educational materials by topic, by the intended primary user (e.g., provider guides for patients, patient self-use) and by type of material.
- Materials for patients to use to educate themselves should contain the following information:
- The importance of screening.
- Type of screening tests, if options exist, such as in colorectal cancer screening, and how the test is done. Materials may also focus on a single type of test.
- Frequency of screening.
- The benefits and potential risks of screening.
- Likelihood of out-of-pocket costs.
- Common misperceptions on cancer screening.
- Materials may be organized by type:
- Printed materials, including photo novellas and graphic materials.
- Materials for mail-based outreach and education.
- Materials for social media-based outreach and education.
- Web-based materials, including downloadable materials.
- Video-based materials.
- Other materials.
3. Tailor your approach using culturally appropriate materials, messages and messengers.
It is useful to remind the team of the common elements and approaches involved in tailoring educational content and messaging for any population of focus. This includes:
Language and tone:
- Developing materials in language(s) spoken by the population of focus.
- Using simple language free from medical jargon. Ensure readability and comprehension of material for all education levels.
- Ensuring that the tone is empathic, respectful and culturally sensitive.
- Using familiar concepts for the population of focus, including incorporating familiar symbols, metaphors and analogies that resonate with their culture(s).
Use of visuals: Use images, familiar symbols, graphics, illustrations, video and/or other visual media that are culturally relevant to the population of focus.
Incorporate storytelling:
- Use personal stories from members of the population of focus who have undergone screenings to emphasize the benefits.
- Share success stories of community members who have undergone screenings to encourage others.
Identify the most appropriate messengers and mechanisms: This includes asking and answering the following questions:
- Who are the best people or groups to get the message across? Who can speak to our subpopulations in their primary language? Should we partner with others for this?
- What communication mechanisms are most appropriate for the population of focus?
- Are there culturally significant events or holidays to convey the message of prevention and care?
- Where should we distribute these materials (e.g., practice waiting areas, community events, local organizations trusted by the population of focus, etc.)?
Accuracy and alignment with clinical guidelines: This involves having changes to materials reviewed by experts to ensure the changes adhere to clinical guidelines.
4. Put formal and informal feedback loops with patients and the care team in place.
Suggested team member(s) responsible: QI lead or their designee.
To help ensure that educational materials are meeting the needs of patients and the processes involved are consistently feasible for the care team, it is important to have both formal and informal feedback loops.
For patients, feedback loops might include:
- Sharing materials with the population of focus to gather feedback and ensure accuracy before testing.
- Getting feedback from patients directly after testing materials or a new approach with them, and incorporate their feedback into your next test.
- Patient satisfaction surveys (or similar).
- Follow-up calls with a subset of patients to understand what works well and what could be improved.
- Patient focus groups.
- Having the practice’s patient advisory board (or similar) provide feedback.
For the care team, feedback loops might include:
- Existing or new staff satisfaction and feedback mechanisms.
- Regularly scheduled meetings and calls to get staff feedback on processes, methods and tools.
Implementation tips
- Borrow from other health centers but be sure to test aspects in your own community. Language, materials and techniques may resonate better in one community than another.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
KEY ACTIVITY #11:
Provide or Arrange for Cancer Screening
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; pre-visit planning and care gap reduction; care coordination.
Overview
This activity provides guidance for setting up a cancer screening test for colorectal cancer, breast cancer and/or cervical cancer for any patient eligible and due for screening who agrees to complete the recommended screening. This includes patients who agree to get screened during a patient visit, in response to a screening reminder, or in response to an outreach activity. This includes guidance on the following screening methods:
- Screening at the practice.
- Providing screening materials during the visit to take at home.
- Providing screening modalities outside of the practice (e.g., mailed FIT, self-referred mammogram, or setting up an appointment for colonoscopy).
- Utilizing community linkages for screening (e.g., providing patients with contact to a mammography center or an outpatient surgical center for colonoscopy or sigmoidoscopy).
This activity includes tailored messaging and a choice in screening modalities, where feasible, to increase the likelihood that populations who have historically had lower cancer screening rates, higher rates of certain cancers and/or higher mortality rates, complete the recommended screening. Initiating a screening recommendation provides an opportunity for a dialogue with patients that may uncover barriers to beliefs about screening and the ability and interest in undergoing screening, including the opportunity to identify unmet social needs that are a barrier to getting screened. Our guidance for this activity, therefore, includes assessing social needs and addressing them.
As discussed in Key Activity 3: Use Care Gap Reports or Registries to Identify All Patients Due for Cancer Screening, Key Activity 6: Conduct Proactive Outreach to Patients Due for Screening, and Key Activity 8: Refine and Implement a Pre-Visit Planning Process, technology can support care teams in their efforts to address cancer screening. Each modality will draw upon similar technical enablers to facilitate cancer screening for adults. The format in which planning is done needs to consider the workflow and staffing model.
Also, as discussed elsewhere, linkage to other sources of screening or follow-up is ideally facilitated by direct data connection to EHR and care coordination applications to support referral and follow-up, but where this is not possible a more manual process ought to be accompanied by a registry like function. (See the next section.) Relevant HIT capabilities to support this activity include care guidelines, registries, clinical decision-making support, care dashboards and reports, outreach and engagement, and care management and care coordination.
Action steps and roles
1. Plan for the cancer screening.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening.
Planning and preparation are covered in the following key activities:
- Key Activity 2: Develop or Update the Practice’s Cancer Screening Protocols.
- Key Activity 3: Use Care Gap Reports or Registries to Identify All Patients Due for Cancer Screening.
- Key Activity 5: Develop and Implement Standing Orders.
- Key Activity 8: Refine and Implement a Pre-Visit Planning Process.
- Key Activity 9: Partner with Patients to Discuss Cancer Screening During Patient Visits.
2. Provide or arrange for the selected cancer screening.
Suggested team member(s) responsible: Designated care team member, based upon the test and the practice’s standing orders.
This is always based on your practice’s clinical practice guidelines and cancer screening protocols (See Key Activity 2: Develop or Update the Practice’s Cancer Screening Protocols).
- Based upon the screening indicated and the option selected by the patient, if choices are available, the appropriate care team member will implement the practice’s cancer screening protocol and standing orders.
- If the selected screening test can be done during the visit, the clinician will implement the screening process, such as a Pap smear or fecal testing.
- If the selected screening procedure is to be conducted by the patient at home using a self-collected sample (e.g., FIT kit), the clinician will explain and demonstrate the proper collection techniques and the process to return the specimen for testing.
- If the screening requires further appointments (e.g., mammogram, colonoscopy, Pap Smear), the medical assistant or appropriate care team member may schedule the patient in the practice or initiate the referral, per protocol, and schedule the necessary follow-up appointments or provide referrals using preestablished community linkages.
- If the screening test requires laboratory analysis (e.g., Pap smear, fecal testing), the clinician will explain when the patient can expect to receive the results and what to do next.
3. Document the screening.
Suggested team member(s) responsible: Medical assistant.
The medical assistant documents the cancer screening test(s) selected, including test type(s), date(s), and any relevant notes, in the patient's electronic health record (EHR).
FIGURE 19: EXAMPLE OF SCREENING DOCUMENTATION
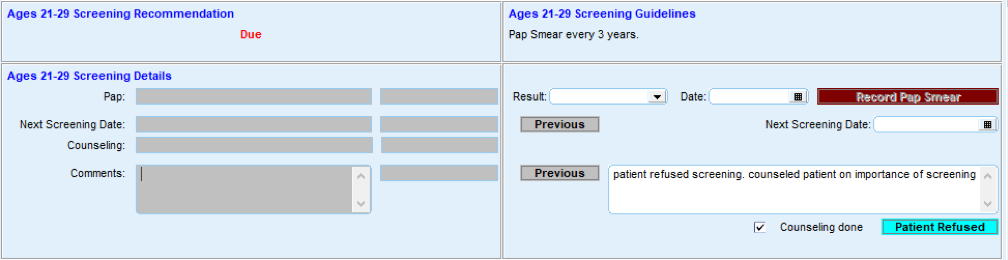
4. Conduct ongoing quality assurance of the cancer screening process.
Suggested team member(s) responsible: QI lead or their designee(s).
The QI lead or their designee(s) should develop and implement a process to review a subset of records each month to ensure that this practice is in compliance with the practice’s cancer screening protocols, standing orders and any other of the practice’s relevant policies and procedures. In cases where policies and procedures are not being followed, the QI lead should initiate corrective action.
Sample workflow for colorectal cancer screen using FIT test
- Assure that the patient is eligible for fecal-based testing by referencing your organization’s screening protocols outlined in Key Activity 2: Develop or Update the Practice’s Cancer Screening Protocols.
- Provide culturally and linguistically appropriate education materials and decision aides to the patient to ensure that they understand the benefits, risks and follow-up schedule for fecal-based testing (see Key Activity 10: Use Culturally Appropriate Education Materials for Cancer Screening). Best practices include:
- Informing patients about the ability of different tests to detect colon cancer.
- Noting the frequency of each test type.
- Providing the patient with testing materials and instructions for the test of choice.
- Highlighting the time frame to return the sample.
- If the patient is available on site, review the sample procurement technique, return system and time frame with the patient while at the practice.
- Using culturally and linguistically appropriate materials and providing graphic diagrams where possible.
- If the patient is receiving the testing materials by mail, provide culturally and linguistically appropriate educational materials with an emphasis on graphic instructions and include information and materials for returning the test to the practice. Invite the patient to contact a specific person at the practice, if they have any questions.
- Document the delivery of materials for testing in the EHR.
- Process the sample or send the sample for processing within 24 hours of receipt and record the results in the EHR.
- Document results and instructions in the EHR. Assure that the EHR cancer screening report notes the patient as having received a fecal-based test in order for the eligibility roster to be generated in accordance with the USPSTF testing frequency guidelines.
- Contact the patient to inform them of the test results and the recommended follow-up (see Key Activity 12: Develop and Implement a Follow-Up System for Those Who Have Been Screened).
- If the patient cannot be reached to communicate positive test results, the team should design and implement alternative methods to reach patients, such as deploying community health workers to attempt to reach the patient and/or sending written communications.
Sample workflow for use of a mobile Pap screening clinic
- Front office staff contact the patient by phone or in person to establish an appointment for a Pap smear and affirm the patient’s knowledge that the appointment is for the mobile unit. The front office staff confirm the patient’s understanding of the date and location of the appointment and how the mobile unit can be identified.
- Front office staff send the patient confirmation of the appointment by mail or text message with the date, time, and location and a reminder of the appearance of the mobile unit. They provide a number to call for questions or changes ahead of the appointment.
- The front office staff or mobile unit staff confirm the patient’s availability by text message the day before the appointment.
- At the mobile unit site, the clinical support personnel, such as an MA, greet the patient and invite the patient to enter the unit for pre-visit information gathering and confirmation of the appointment purpose and prepare the patient for the exam.
- The clinician conducts the exam, secures a Pap Smear, and addresses other clinical needs of the patient.
- The clinician and/or clinical support inform the patient of when the result is expected and how the patient will be informed of the result.
Implementation tips
- Where applicable, it is critically important to guide the patient in how to obtain and return the sample based on the type of test selected. Timing of the sample’s return is of the greatest importance and going outside the return time frame may render a sample to be invalid.
- Where multiple test modalities are offered, be sure to note the frequency of retesting, based on the method selected by the patient.
- Work with community representatives to develop messages and tools that are culturally relevant and understandable by the population.
- Test the message with community members to assure relevance and cultural accuracy.
- Review the use of the testing materials while the patient is in the practice, if possible, and encourage them to ask questions and review the testing and return technique.
- Encourage patients to complete the test in a brief time frame so that it remains top of mind.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
KEY ACTIVITY #12:
Develop and Implement a Follow-Up System for Those Who Have Been Screened
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; implement condition-specific registries; proactive patient outreach and engagement.
Overview
This activity outlines the importance of communicating screening tests results to patients and initiating timely follow-up on positive results.
Screening for colorectal, breast and cervical cancer is performed to detect cancer early and to enable early treatment to prevent advancement of the cancer and improve survival. Patient safety is advanced when providers use a closed loop strategy to communicate results. Positive screening test results – evidence of cancer or suspicions of cancer [24] [25] – show that timely follow-up is optimal and patients prefer that positive results be communicated swiftly and verbally.
California does not require a provider’s order for a person to obtain a screening mammogram, but it does require that the mammography provider notify both the patient and their healthcare provider of the results of screening mammography. Consequently, the follow-up system should include a process for receiving mammography results, communicating with the patient following the results, and implementing a conversation with the patient about the need for further follow-up if the results are positive or suspicious.
Low-income and minority populations have higher death rates from breast, cervical and colorectal cancer and experience inequities in access to care to address cancer. When patients agree to undergo screening tests, timely communication of test results improves rapid follow-up of positive tests and supports effective communication with the healthcare system. When the patient understands the test result and its meaning and they are given the opportunity to understand the implications of the result, they are more likely to engage in follow-up.
Follow-up of test results, positive or negative, empowers patients with information that supports their ability to make decisions about future screening or diagnosis. While many systems have patient portals, active notification of test results allow patients with significant social needs to understand the implications of those results. Patients can then access supportive services that improve their ability to follow through with further care when screening tests are positive.
Most EHR platforms allow for the identification of outstanding orders and referrals. When an order or referral is placed, it remains open until that service is completed. Leveraging a report on open orders and referrals for this intervention will allow for reconciliation of those orders. Outreach to patients will allow for determination of the status of the test or referral, provide an opportunity to remind the patient, get feedback on their intention to follow through, or allow for reconciliation with the testing or referral site.
Where possible, the use of external data (e.g., claims, HIE data) can be leveraged to minimize individual patient outreach efforts. The use of patient engagement technologies can also be leveraged as indicated.
Action steps and roles
1. Develop or refine your practice’s screening follow-up protocol.
Suggested team member(s) responsible: Panel manager and their designee(s).
The multidisciplinary team will start by identifying the person or persons who will be responsible for creating a protocol for follow-up of screening test results. In many cases, this will involve the panel manager and other members of the care team. This team will create a protocol that allows care team members to provide a prompt response and to notify the PCP when a test result is positive. This process must use closed loop communication where every test result is sent, received and acted upon in a timely manner in accordance with the practice’s protocol.
At a minimum, the protocol will identify:
- Which types of screening tests will require health center follow up, such as fecal-based testing and Pap smears.
- Who (specifically) will provide the follow-up. For example, colonoscopy follow-up is typically provided by the clinician who conducts the colonoscopy.
- How the follow-up will occur, the timing of the follow up, and the language in which to communicate the results, which may be constructed through the EHR. For example, the team may elect that normal screening test results will be communicated by mail while positive results will be followed up through a telephone call from a clinician or a direct office visit to enable the patient to ask questions and receive guidance.
How results are communicated is critically important. A Preventive Medicine Reports article in 2019 references a systematic review of communication methods used to deliver results of cancer screening. They found that several aspects of the communication were important:
- Timeliness of the report.
- Accuracy.
- Visual support.
- The ability to ask questions.
- The privacy of the results.
- Managing expectations.
2. Implement your practice’s screening follow-up protocol.
Suggested team member(s) responsible: Care team member indicated in the practice’s protocol.
- Share results and initiate follow-up steps, if needed. This process must use closed loop communication where every test result is sent, received and acted upon in a timely manner in accordance with the practice’s protocol. The care team member tasked with screening test follow-up will create a communication to the patient.
- Normal screening results may be shared in writing or in a follow-up visit with the patient. Patients should be informed when the next screening is due.
- Abnormal or suspicious findings should be communicated by telephone call or in an office visit directly with the provider.
- When the patient cannot be located or a visit is missed, there should be a system to increase attempts to reach the patient with the results.
- If the patient appears in the health center, abnormal results should be flagged in the record and communicated with the patient.
- Attempts to reach the patient and share the results should be documented in the record.
- Discuss the results with the patient. Clinicians should be prepared to respond to discuss the implications, invite patient questions, discuss the need for and type of follow-up, and be ready to offer the team’s assistance in organizing the follow-up.
- Abnormal results should be explained to the patient and allow an opportunity for the patient to ask questions. Guidance from the National Cancer Institute and CDC[26] can help to explain the results to the patient and assist them in understanding the recommended follow-up to the Pap smear based on the Pap smear result.
- Follow-up of the abnormal results should be scheduled as soon as possible with the direct assistance of practice staff.
- Patients should be offered care coordination or supportive services to enable them to address social and other needs that interfere with their ability to follow through on further care.
- Maintain contact with the patient to help ensure follow-up. The care team should maintain contact with the patient to help ensure that they engage in any recommended follow-up and that they continue to receive any recommended follow-up, if the screening test was positive, and future screening(s). This can be accomplished by telephone, electronic reminders or mailed reminders.
- Document the result. The team member who initiates the communication of the test result documents the result, communication of the result, and the recommended follow-up in the EHR.
Example workflow
Based on the type of screening test and your practice’s screening protocols, your workflow may differ.
- Results are communicated to the practice.
- Per the practice’s protocol, test results may be divided into “within normal limits,” “positive,” "suspicious” or “abnormal.”
- If the screening test was performed by an outside provider, such as a mammography center or a surgical center, the screening entity may provide the results to both the patient and to the practice.)
- Results are communicated to the patient.
- Normal results are communicated to the patient via telephone, letter or the EHR portal or in person.
- Abnormal, positive or suspicious results trigger direct communication with the patient to make an appointment to discuss the results with the PCP or, per protocol, with another team member designated to follow up.
- If the practice uses a portal to identify test results, the practice will need to determine if and how positive results are posted in the portal to enable patients to obtain information as soon as possible.
- During the visit, the PCP discusses the results with the patient, explains the recommended follow-up and responds to the patient’s questions.
- Where indicated, a follow-up appointment is initiated with a practice provider.
- Where indicated, an appointment is initiated with an outside provider and the appointment is shared with the patient.
- The referring clinician initiates a referral letter to the outside provider to provide information about the patient.
- A practice team member may be tasked to follow up on appointments to identify if appointments were kept.
Implementation tips
- Use the EHR to support standardizing language for negative test results (within normal limits) in electronic or mailed communications.
- Recognize that communicating positive or suspicious results may result in anxiety on the patient’s part. Therefore, time the communication so that the practice can be reached by telephone when the patient is likely to receive the results.
- Offer patients an opportunity to discuss test results by phone or in an office appointment, even if the result is negative.
- Follow up on abnormal results if the patient is not easily reached by using community liaisons and adhering to Health Insurance Portability and Accountability Act (HIPAA) guidelines.
Evidence base for this activity
Williamson S, Patterson J, Crosby R, Johnson R, Sandhu H, Johnson S, et al. Communication of cancer screening results by letter, telephone or in person: A mixed methods systematic review of the effect on attendee anxiety, understanding and preferences. Preventive Medicine Reports. 2019 Mar;13:189–95.
KEY ACTIVITY #13:
Coordinate Care
This key activity involves the following elements of person-centered population-based care: proactive patient outreach and engagement; pre-visit planning and care gap reduction; care coordination; behavioral health integration; address social needs.
Overview
This activity will help you provide care that is patient-centered and coordinated across all internal and external providers involved with the patient.
Care coordination involves deliberately organizing patient care activities and sharing information among all participants concerned with a patient’s care to achieve safer processes and more effective outcomes.
Care coordination is an integral part of providing high-quality population-based care in an ambulatory care clinic. In your practice, care coordination happens daily as part of standard work and includes activities such as assisting patients with referrals, managing patient messages and receiving incoming information from outside care partners, including pharmacies, specialists, hospitals and community partners (e.g., schools, employers and community-based organizations). For perinatal patients, care coordination also involves intentional communication pathways between the Community Health Center (CHC) and hospital system responsible for intrapartum care.
Managing care across multiple settings is often a source of frustration and dissatisfaction for both patients and providers. Lack of coordinated care can result in both duplication of services and lack of completion of needed services. When care is not coordinated, decisions can be made without a complete picture of the patient’s needs and goals. Practices that have effective care coordination processes often employ “closed loop referrals” as a best practice.[27][28] In a closed loop referral, a referring provider can track the status of the referral (completed or pending) as well as the outcome of the referral, including whether additional follow-up is needed. When closed loop referrals are implemented, both provider and patient satisfaction is enhanced.
There are four fundamental concepts when thinking about care coordination activities within your practice:[29]
- Accountability | A key to successful care coordination is accountability. Clinics and systems that excel at care coordination take ownership and assume responsibility for care coordination activities. Examples of care coordination processes include development of infrastructure for care coordination, identification of staff responsibilities, and relationship building with referral partners. Together, these actions create the ability to support referrals and transitions and track them to completion.
- Patient support | Referrals and transitions often raise questions for patients and families, and can be a source of anxiety. Not only do the logistics of a referral or transition create questions, but also patients and their families can experience anxiety regarding the outcome or purpose of a referral or transition. When a practice can dedicate staff time to addressing the needs of patients and families, referrals and transitions are most likely to accomplish intended goals.
- Relationships and supports | Referrals and transitions work best when all parties – patients, primary care providers, and consultants – agree on the purpose and importance of the referral and the roles that each will play in providing care.
- Connectivity | Build relationships and agreements among providers, including community agencies, that lead to shared expectations for communication and care. Connectivity includes developing referral relationships, accepting accountability and creating data flow.
In order to conceptualize how practices can coordinate with community-based agencies (housing, food, income support), hospitals and ERs, and specialists, refer to the visual depiction of the care coordination model by the Center for Accelerating Care Transformation (ACT Center).
Care coordination can benefit all patients in your practice, and care coordination processes, such as closed loop referrals, can be applicable to many practice activities (see examples in the table “Care Coordination Quality Improvement Strategy” below). Common areas where care coordination can be enhanced include specialty referrals, behavioral health referrals and connections to community resources such as WIC and paid family leave.
Care coordination activities can also identify patients who need a higher level of support through a care management program (see Key Activity 17: Provide Care Management). Care management differs from care coordination in that a patient consents to and works directly with a care manager to create a care plan that is shared with an extended care team. While a practice can decide whether it has the resources to provide higher level care management services, care coordination is a foundational activity of effective population health management.
Care coordination for Medi-Cal members
Patients with Medi-Cal have access to many resources that can support improved health outcomes and social needs. These Medi-Cal resources can be another opportunity to create care coordination processes in your practice; see Figure 20 for a partial list of Medi-Cal programs that involve care coordination. As of 2023, both doula care and community health worker services are a benefit of the Medi-Cal program. The services of CHWs (also known as Promotores/Community Health Resources) can be highly effective in helping to connect patients to resources and educational materials that are community-based and aligned with a patient’s values, language and cultural needs to further health equity. The California Health Care Foundation offers an overview of the CHW program, including potential hurdles.
Medi-Cal patients with high medical, behavioral health and/or social needs may be eligible for Enhanced Care Management and Community Supports (see the going deeper Key Activity 17: Provide Care Management for more information on these programs).
There are also a number of Medi-Cal programs that provide services and support to Medi-Cal patients who qualify. Figure 20 provides a high-level overview of care coordination opportunities.
FIGURE 20: EXAMPLES OF MEDI-CAL PROGRAMS OR BENEFITS THAT INVOLVE CARE COORDINATION
- Enhanced Care Management (ECM)
- Community Supports
- Community Health Workers
- Doula
- Long term services and supports (LTSS)
- California Children’s Services (CCS)
Effective care coordination helps your practice to better meet the broad range of patients' needs and preferences. It helps ensure that each patient's needs and preferences are known and communicated at the right time to the right people and that this information is used to guide the delivery of safe, appropriate and effective care.
Key outcomes of care coordination:
- Greater patient participation and satisfaction.
- Closed gaps and addressed inequities that exist in access to care.
- Improved quality outcomes and the potential to leverage quality financial incentives.
- Decreased staff burnout and duplication of services (e.g., “Let the system do the work”).
Effective care coordination helps to reduce health inequities among different populations by better identifying and then working to address a broad range of patient needs. When care coordination is effective, the patient is at the center of care and the patient's cultural, social and health needs are incorporated into a plan of care. By identifying outside clinical supports (e.g., hospital care, specialist care) and social supports (e.g., housing assistance, transportation, food assistance, etc.) and creating pathways for coordination, the practice promotes equity by facilitating access to these services for all patients who need them. As part of a care coordination strategy, practices should consider the growing number of aggregator organizations (see resources section below) that partner with community-based programs and resources centered around addressing social needs, such as housing, food and community. Many of these community-based programs are tailored for specific demographic groups and can support patients in meeting their needs in a culturally competent way.
Technology can be used to streamline and enhance care coordination. Care coordination technology tools can support care coordination activities, such as registry, clinical decision support, care gap reporting, and patient outreach engagement technology.
Practices can use a care coordination self-assessment tool, such as those referenced in the action steps below, to determine if additional technology support would be indicated.
Registry and care gap reporting
Practices can use an analytics platform to identify patients and group patients who have similar needs together. Your practice can then tailor interventions to meet the needs of a population. For example, a practice can use data to identify those people who have not had a visit in the past year or those who are due for colon cancer screening. Data may come directly from the medical records or from claims data that is submitted to the payor.
Care coordination software: Clinical decision support
Many EHRs have care Coordination modules, which can be used to develop assessments and care plans. Alternatively, care coordination modules can be added on to an EHR. Care coordination software can support assessment, referral and coordination pathways (e.g., clinical decision-making support). Using a care coordination software module, a practice can develop an assessment with questions regarding a patient’s social health needs. If answers are positive, the software can be configured to provide referral pathways and prompt care coordination activities. For example, if a patient answers “yes” to questions on food insecurity, the assessment can prompt referral to local resources, such as food banks, meal programs and nutritional supports. The system can also prompt the staff to reassess progress at the next visit.
For more information, see Appendix E: Guidance on Technological Interventions.
Action steps and roles
The action steps and roles outlined below provide a framework for assessing current state, developing a strategy, implementing care coordination, and monitoring and learning as you progress.
1. Establish a call to action for care coordination.
Suggested team member(s) responsible: Medical lead, care coordination staff, QI staff.
To achieve effective care coordination, your practice needs to see yourself as a hub or center for coordination of patient care.
- While all providers within a patient’s support network need to collaborate, your practice should view itself as accountable for care coordination for empaneled patients and patients who have been assigned to your practice by their managed care plan.
- Identify a care coordination team who will be responsible for leading the evaluation, assessment and implementation of care coordination activities.
- Share with the care coordination team why care coordination is important.
- Some areas to address:
- What is care coordination?
- Why does care coordination benefit staff and patients?
- Review proposed framework (assess, develop strategy, implement, monitor) to develop the care coordination activity.
- Identify and share some initial projects directed at enhancing care coordination.
- Invite and engage staff and patients to provide input on how they think care coordination can be enhanced.
- Discuss how to best incorporate feedback from patients.
- Use feedback from this activity and incorporate it into assessment.
2. Assess your practice’s current state of care coordination activities.
Suggested team member(s) responsible: Panel manager and QI lead.
Begin by asking and answering the following questions:
- What does the practice hope to achieve by implementing interventions to change the care coordination process?
- Which patient needs are the responsibility of the practice to provide care coordination within the practice)?
- Which patient needs will need to be addressed through external referral and collaboration (e.g., care coordination with entities outside the practice)?
- What resources are needed to improve the current state (data, input from care team members, input from care partners such as hospitals, specialty, behavioral health and social needs)?
Gather stakeholder input:
- Conduct interviews or surveys with patients, community members, care and case managers, community support providers and community-based organizations that have referral pathways to Medi-Cal managed care plans (MCPs), such as Comprehensive Perinatal Services Program (CPSP), home visiting programs, etc. to understand their perspectives on current care coordination processes, challenges, and areas for improvement.
- Conduct interviews with your care team to understand their perspectives on current care coordination processes, challenges and areas for improvement.
Analyze existing systems and processes:
- Review existing care coordination protocols, technologies and resources utilized by health centers.
- Explore how technology is currently being used to support care coordination. Then, explore how technology could be harnessed to streamline care coordination, improve data sharing, and/or enhance communication among providers.
- Review existing formal and informal linkage agreements.
- Conduct an analysis to determine patient needs not yet fully addressed by existing agreements.
- Assess data on patient outcomes including process outcomes (e.g., obtaining labs and imaging as recommended by providers; adherence to recommend prenatal and postpartum follow-up visits); clinical outcomes (e.g., reduction in C-sections, etc.); utilization outcomes (e.g., reduction in ED visits and hospitalizations); satisfaction; and any existing quality improvement initiatives.
- The following resources can help your practice assess its current state:
- Population Health Management Capabilities Assessment Tool (PhmCAT): Sections on population-based care, social health and behavioral health.
- Care Coordination Maturity Assessment: This resource addresses multiple domains that may be impacted as care coordination activities are undertaken.
3. Develop and implement a care coordination improvement strategy.
Suggested team member(s) responsible: Panel manager and QI lead.
Develop your high-level care coordination improvement strategy.
- The high-level strategy is based on the results and insights from your care coordination assessment and your practice’s capacity (bandwidth).
- Establish clear objective.
- Define specific, measurable, achievable, relevant, time-bound, inclusive, and equitable (SMARTIE) goals for enhancing care coordination.
- Ensure that these goals align with the overall mission and values of your practice.
- Prioritize areas for improvement. Identify a limited set of key focus areas, such as communication protocols, technology integration, staff training or patient engagement strategies, based on the assessment findings and your practice’s bandwidth.
Identify resources and infrastructure.
- As part of the implementation of the strategy, identify the resources and infrastructure needed to support care coordination at your practice.
- We have included in Figure 21 below some considerations by resource as you develop your care coordination implementation plan. Some of the resources may not apply to your practice.
FIGURE 21: CONSIDERATIONS FOR IDENTIFYING CARE COORDINATION (CC) RESOURCES
Resource |
Considerations |
|---|---|
Staff |
Dedicated time for the development, training and implementation of CC activity. Define ongoing staffing needs. |
Internal tools |
Electronic health record modifications, data needs and technology needed to implement CC activity. |
Process |
Development of workflows, training materials, communication plan and job descriptions. Identification of what CC activities will be the responsibility of the practice staff. |
Funding and finance |
Explore benefits, such as Enhanced Care Management or community health workers, as well as financial resources that may be available through external partners, such as health plans or hospitals. |
Clinical decision-making support |
Medical staff, behavioral health and social work staff, and patients as subject matter experts (SMEs). |
External support and data |
Information and resources required from the entity with which the practice is coordinating (e.g., hospital, specialist, social health provider). Data exchange requirements, including the new California Data Exchange Framework (DxF), and privacy. |
Develop staffing for care coordination.
- Determine who at your practice will be involved in care coordination. For many organizations, staff involved in the Comprehensive Perinatal Services Program will be key contributors, including ECM lead care managers, particularly the ECM birth equity population of focus.
- PHMI Care Teams and Workforce Guide Resource 1: Core and Expanded Care Team Functions, Team Members and Roles provides an overview of care coordination using a care coordinator or referral manager.
- Based on the population of focus and the intervention, develop staffing requirements and any job description changes to embed care coordination into your practice.
- The responsibilities of a person or people fulfilling care coordination roles should include, at a minimum:
- Manage the referral process.
- Assist patients with transitions, such as those to and from hospitals and other institutions.
- Help patients resolve logistical, financial and/or other barriers to a successful referral.
- Link patients with community resources.
- Follow up with patients within a few days of an emergency room visit, hospital discharge or discharge from a treatment facility. This process should include the transitional care services (TCS) lead care manager during transitions of care.
- Ensure the safe transfer of patient data.
- Track progress (e.g., referral milestones).
- Assist patients who are having difficulties.
- Schedule follow-up and specialty appointments for and with patients.
- Formalize this role through a job description or position description.
- Provide regular training and support to care coordinator or referral coordinator or similar.
Develop or refine your policies and procedures for care coordination. At a minimum, include all of the following:
- Identifying patients who are eligible for care coordination.
- Defining the activities that are associated with care coordination (e.g., outreach, engagement, assessment and desired outcome).
- Managing the referral process.
- Ensuring the safe and timely transfer of patient data.
- Tracking progress through closed loop referral (see referral milestones above).
- Identifying patients who would benefit from higher levels of care coordination, such as care management interventions like Enhanced Care Management (ECM) (see Key Activity 17: Provide Care Management).
Develop or refine your referral protocols.
- Develop clear guidelines for when and how to make clinical referrals.
- Include criteria for urgent versus nonurgent referrals.
- For each provider your practice will be referring to:
- Identify the information, format and process the provider requires to receive a referral.
- Share the information you will need back from the provider when the referral has been successfully made and/or once the service or support has been provided to ensure continuity of care and nonduplication of services.
- Formalizing this through a written agreement or compact is a good way to ensure expectations on both sides of a referral are understood.
- In working with community agencies or organizations that provide social support, the specific information that can and should be shared will depend on the service. It is important to have a mutual understanding about that information, using a written agreement where possible. At minimum, if the referral did not include a warm handoff, there should be an expectation that the practice hears back from the organization to let them know whether the patient followed up on the referral. Sample primary care checklist suggestions for assessing referral process.
Address connectivity. For any providers, patients, family and caregivers who you are exchanging patient data with, your practice should develop formal agreements and processes. Here are some considerations to facilitate information sharing among providers involved in an individual’s care.
- Integrate documentation platforms where possible and standardize how documentation is included in patient records.
- Ensure that behavioral health records are integrated into the overall EHR system to the extent permitted by law.
- Enable providers to access comprehensive patient information.
- Identify and assess participation in health information exchanges and/or social health information exchange systems where feasible to facilitate seamless sharing of patient information between clinicians. These exist in some communities and will develop over time. This explainer series from the California Health Care Foundation describes the new statewide health information exchange coming into effect in 2024.
- Understand when patient consent is required to ensure compliance with privacy regulations, such as the Health Insurance Portability and Accountability Act (HIPAA). Include consent processes in the referral protocols.
o Set expectations about the information that should be shared and develop processes for facilitating that exchange. As data that is used in a clinical setting will likely be different than information that is shared by community agencies or social support networks, it is imperative to have clear parameters around what types of information can be exchanged between your practice and your partners.
Provide care coordination to patients.
- Provide care coordination to patients who need it in accordance with your care coordination policies and procedures and referral protocols.
- Embed care coordination into your practices daily workflow, including pre-visit planning. See Key Activity 8: Refine and Implement a Pre-Visit Planning Process.
- Provide referrals to care management as discussed in Key Activity 17: Provide Care Management, such as ECM for patients who require more intensive support.
- Discuss each referral with the patient to ensure the patient understands the reason for the referral.
FIGURE 22: EXAMPLE OF A CARE COORDINATION QUALITY IMPROVEMENT STRATEGY
Area of Improvement |
Goal |
Why It is Important |
|---|---|---|
Development of registries and a process to outreach to empaneled patients who have not engaged in care. |
Bring individuals into care and screen for social health needs and barriers. |
Supports increased access to preventive medical care and addresses barriers related to social health. |
Development of a closed loop referral pathway for social services when social health barriers are identified. |
To support patients receiving services that will be beneficial to address social health barriers, including those that are culturally aligned and based in communities where they live. |
Health outcomes, including health equity and access, are improved when social health needs are met. |
Development of a referral pathway to specialty providers and the creation of a pathway to receive consult results. |
To optimize access to specialty services, including urgent referral, and to ensure consultation results are available to the PCP. |
To ensure timely access to services, including urgent referrals. Receipt of consult information saves time and prevents duplication of services. |
Development of a process to follow up with patients after transitions of care, post hospitalization. |
To support optimal outcomes for patients post hospitalization and decrease the chance of rehospitalization. |
Transition from the inpatient (hospital) setting back to home often results in poor care coordination, which can lead to medication errors, repeated or incomplete diagnostic workup, and lack of clarity regarding what a patient needs post hospitalization. |
The following resources can help your practice develop your care coordination improvement strategy:
- NCQA Care Coordination Webpage.
- HEDIS Measures: Transitions of Care.
- HEDIS Measures - Follow-Up Care After.
4. Monitor continuous quality improvement of care coordination.
Suggested team member(s) responsible: QI lead.
- Develop both formal and informal mechanisms to obtain feedback from providers, staff and patients on the effectiveness of your practice’s care coordination efforts and to identify areas for improvement.
- Establish key performance indicators related to care coordination. Consider using the Agency for Healthcare Research and Quality (AHQR)’s Care Coordination Quality Measures in Primary Care (CCQM-PC). Your performance indicators will vary based upon your improvement strategy and the needs of patients, but may include metrics, such as:
- Number of adults with preventive care needs engaged in care.
- Number of adults with preventive care needs who are up to date on their screenings.
- Percent of closed loop referrals by referral entity. Monitor and analyze metrics to gauge the effectiveness of your internal systems and how well each referral partner is promoting coordination and collaboration.
- Continue to build relationships with providers and community support agencies that are available to provide services to CHC patients.
- Consider developing and using a care coordination variance report. Here is a sample patient care coordination variance report.
Implementation tips
- An interoperable referral management system can help practices streamline the intake process, communicate with other providers and social service agencies, track the status and outcomes of referrals, and ensure that patients receive the appropriate level of care and support.
- Leverage community health information exchanges (HIE) or Cal HHS Data Exchange Framework Data Sharing Agreement to gain access to admission, discharge, and transfer information. Absent being able to access an HIE, establish relationships with your local hospitals to be able to gain daily notification of your patients being discharged.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
KEY ACTIVITY #14:
Use Social Needs Screening to Inform Patient Treatment Plans
This key activity involves the following elements of person-centered population-based care: behavioral health integration; address social needs.
Overview
This activity provides guidance on screening patients for health-related social needs and how the information can begin to be used to inform patient treatment plans, including referral to community-based services. Social needs are defined as individual material resources and psychosocial circumstances required for long-term physical and mental health and well-being, such as housing, food, water, clean air, sanitation, and social support.
Evidence continues to accumulate that demonstrates not only the ways in which social needs impact physical and mental health outcomes[30][31][32] but also how worsening physical and mental health conditions can impact social stability.[33][34] Social barriers also have a significant impact on patients’ ability to carry out preventive health measures, such as recommended breast, colon and cervical cancer screenings. Common barriers include financial insecurity, housing insecurity, transportation, language, health literacy, behavioral health needs, cultural beliefs, parenting responsibilities and fear of test results; each affect a patient’s ability to prioritize and carry out recommendations for screening. Unless these barriers are understood and addressed, patients will not be able to carry out the testing.[35]
Screening for social needs provides an opportunity to ensure healthcare is provided in the context of life circumstances in which patients and their families are living and part of providing holistic, person-centered care.
Health equity is advanced by addressing the underlying issues that prevent people from being healthy. At the population –level, this means addressing communitywide social drivers of health and structural determinants and at the individual level this means acknowledging and beginning to address health-related social needs.[36]
Social needs screening often asks questions about private and potentially stigmatized aspects of a patient’s life (e.g., poverty, interpartner violence).[37] See below about how to screen for and respond to social needs in a trauma-informed way. Be aware that staff may also experience trauma when screening for social needs (e.g., if a staff person has a history of food insecurity or experiencing homelessness in the past) and have a plan to support staff needs.
Note that there is not yet consensus or uniform endorsement regarding screening for social needs due at least in part to a lack of high-quality evidence on the risks, benefits and best practices of screening and response.[38] For example, findings from the recent Centers for Medicare and Medicaid Services (CMS) Accountable Health Communities Model indicate that social needs screening and navigation services reduced ED utilization and may have reduced expenditures but did not appear to increase patient’s connection to community services or resolve the patient’s social needs.[39] Other possible ways social needs screening can support attending to social needs include strengthening the patient-provider relationship,[40] destigmatizing social services, tailoring care, and increasing social investment at a population level.[41]
Technology-enabled screening can be utilized to screen patients for social needs through various channels: Directly in the EHR, in applications used by care coordinators, and directly to patients via patient-facing outreach and engagement technologies. Practices will need to determine a process to assure that externally generated screening information is incorporated into the patient record and used for care delivery, risk assessment, and management and for managing relevant referrals. Social health information exchanges exist in some communities to identify where patients are getting social services and help identify higher risk patients; these will develop over time.
Action steps and roles
1. Pick a validated standardized screening instrument and establish how to document results.
Suggested team member(s) responsible: Clinical leadership.
Pick a standardized screening tool that fits your organizational environment and the context of your patient population. The Social Interventions Research and Evaluation Network (SIREN) conducted a review of social needs screening tools and provides a comparison and search tool for different tools for adults and children. Currently available standardized tools you might consider include:
- Protocol for Responding to and Assessing Patients’ Assets, Risks and Experiences (PRAPARE, developed by the National Association of Community Health Centers (NACHC)).
- The CMS Accountable Health Communities (ACH) screening tool.
- REACH by Health LEADS.
- The Academy of Family Practice (AFP) tool.
Work with your clinical informatics team to determine how staff will document screening results in your EHR. Some EHR systems have screening tools already embedded and many are moving quickly to incorporate screening; some EHRs may be able to auto-populate Z codes based on screening results. Your managed care plans may offer additional guidance or support and can be a resource to connect your practice to other organizations who have experience with screening instruments and technology tools. By using the same screening tools and/or information technology platforms, the consistency and quality of care delivery can be enhanced.
For the patient’s problem list, DHCS provides a list of 25 Priority Social Determinant of Health (SDOH) Codes to focus on. This shorter list of codes is intended to capture areas where the health system may have the greatest impact on identifying and addressing social needs.
2. Understand resources and community referrals for positive screens.
Suggested team member(s) responsible: Referral manager, community health workers.
Through CalAIM, the California Department of Health Care Services (DHCS) has taken steps to ensure that Medi-Cal members have access to social support that can impact health outcomes. Connect with your managed care plans (MCPs) and regional area consortia to understand what services and resources they have in place to support patients’ access to community-based social services. Many MCPs are developing relationships with social services agencies to meet the needs of their patients. For Medi-Cal patients with the highest level of social needs, MCPs offer Enhanced Care Management (see Key Activity 17: Provide Care Management) and MCPs offer some or all of 14 CalAIM Community Supports: these community supports provide resources to address social needs, such as housing navigation, recuperative care post hospitalization, and medically tailored meals. Some MCPs use the new CalAssist tool for Medi-Cal members to self-identify whether they are eligible for any of the 14 CalAIM Community Supports and generate a referral to the applicable community support provider.
Many patients who screen positive for social needs will benefit from connection to alternative resources that are not part of Community Supports. Contact your MCP to see if they have established relationships with providers who connect individuals to social services. Health plans may contract with an online provider or with a social services network lead entity that can connect you with existing community-based organizations and public agencies in the area. These contracted services will often include a process for making closed loop referrals where the referring provider is notified if the patient has successfully accessed the referral. Closed loop referrals are a best practice in care coordination that leads to higher levels of patient and provider satisfaction.satisfaction.[42]
Key services to catalog include nutrition assistance, employment readiness, childcare support, postpartum paid family leave, rent and utility assistance, and resources for transportation. Other places to look to build sources for local community referrals include:
- Your current social work staff and/or community health workers, who may have many go-to resources already identified.
- Free online aggregators for local community services, such as findhelp.org.
- A free telephone number providing access to local community services for housing, utility, food and employment assistance.
- Resource networks maintained by a local hospital or larger health system in your area.
For going deeper in this area, practices can consider prioritizing quality improvement activities that establish new or previously underutilized community resources to address specific social needs (see Key Activity 4. Use a Systematic Approach to Address Inequities within the Population of Focus). Case studies provided in the resources linked below provide examples of these improvement initiatives.
3. Provide person-centered care that acknowledges social needs.
Suggested team member(s) responsible: PCPs and the expanded care team.
Information about a patient’s social needs can be used to provide tailored person-centered care and treatment plans that patients are more likely to follow. For example, if a patient screens positive for transportation insecurity, discuss the transportation needs as part of the broader discussion of cancer screening options. Use trauma-informed ways of engaging patients in their own care, including developing shared goals, providing self-management support, and using communication techniques, such as motivational interviewing. (See the resource Trauma-Informed Population Health Management.)
The desires and goals of the patient will inform how and when to move ahead in addressing social needs. It is important to support patients through motivational interviewing and trauma-informed practices to create a person-centered care plan. By having processes in place to support ongoing person-centered care planning, such as a warm handoff to a care coordinator or a documented plan to follow up on issues discussed at the next visit,) will help to build trust and support connection to community referrals when the patient is ready.
4. Establish a workflow for screening and referrals.
Consider screening before the patient meets with the PCP and have a workflow in place for follow-up on a positive screen (e.g., meet with the care coordinator or care management staff who will facilitate referral). Take steps to flag the positive screen so the PCP is aware and can address any positive screens during the visits.
Train staff in the new workflows and how to provide trauma-informed screening. Staff often initially resist screening if they feel they don’t have the tools to help address positive screens.
- Following screening, ask patients for their prioritized needs and whether they would like assistance before making a referral.
- Providing a written script for staff and accompanying signage can build confidence in dealing with the challenging circumstances many patients experience.
See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
Examples from the Center for Care Innovation (CCI)
Case studies of how practices implemented screening tools and undertook innovative quality improvement activities to address food insecurity and transportation insecurity.
Addressing Food Insecurity: Concrete Advice From Clinic Leaders
Food Insecurity: Hidden Hunger in Los Angeles
Addressing Transportation Insecurity: Concrete Advice From Clinic Leaders
Going Deeper Key Activities
More advanced activities that build off the key activities and that help to ensure your practice can achieve equitable improvement in your cancer screening rates.
KEY ACTIVITY #15:
Strengthen Community Partnerships
This key activity involves the following elements of person-centered population-based care: proactive patient outreach and engagement; care coordination; address social needs.
Overview
Strengthening partnerships with community-based organizations that share the practice’s vision and goals for its patient population enables the practice to provide more holistic care to patients and meet crucial patient social needs. Your practice can leverage existing community infrastructure to provide resources unavailable within the medical home. Community partners provide various services that meet patients’ basic needs, such as education, housing, food, transportation, employment assistance and social support.
This activity provides considerations and tips for strengthening partnerships, although note that deep engagement in this work can require significant resources from your practice.
Local social service organizations are rooted within the communities they serve; thus, they have a deep understanding of the needs and preferences of their communities, as well as local resources and challenges. They can support outreach and health literacy efforts and participate in codesign by providing insight around clinical initiatives serving particular patient populations.
Building and strengthening relationships with community-based partners can extend the reach and impact of practices by facilitating patient access to services that the practice is unable to provide. Through partnerships with organizations that provide housing supports, nutrition assistance, transportation, and other social needs, practices can play an active role in helping to address health-related social needs. The PHMI Equity Framework and Approach should inform partnership development to ensure the work of partnership building is prioritizing community groups that experience injustice and structural violence.
Action steps and roles
1. Start by understanding the resources and partnerships available through Medi-Cal.
Suggested team member(s) responsible: Clinic operations and leadership staff who will liaise with community partners.
Resources include supports that are available to all Medi-Cal patients, such as transportation to medical appointments, as well as Medi-Cal Community Supports for eligible individuals with higher levels of social needs. The following is a list of social support resources that are available to Medi-Cal patients when eligibility criteria are met.
- Transportation to medical and other Medi-Cal-covered appointments.
- In-home supportive services, including personal home care assistance for those who qualify.
- Community-based adult services, including day programs outside the home for individuals who need assistance with activities of daily living.
For individuals with the highest needs, the 14 Medi-Cal Community Supports are:
- Housing transition navigation services.
- Housing deposits.
- Housing tenancy and sustaining services.
- Short-term post-hospitalization housing.
- Recuperative care (medical respite).
- Day habilitation programs.
- Caregiver respite services.
- Nursing facility transition and diversion to assisted living facilities.
- Community transition services and/or nursing facility transition to a home.
- Personal care and homemaker services.
- Environmental accessibility adaptations (home modifications).
- Medically supportive food and meals and medically tailored meals.
- Sobering centers.
- Asthma remediation.
For specific providers in your area, contact the provider services department of your Medi-Cal managed care plan to learn more about the providers of these services. With a list of contracted community based organizations, your practice can start to build relationships and support ongoing social needs for your patients.
For an overview of Community Supports in the state, DHCS has provided information about the current state of Enhanced Care Management and Community Supports in the Community Supports Year 1 Summary and the Enhanced Care Management Year 1 Summary.
2. Complete an environmental scan to understand who your patients identify as trusted messengers and resources in their community.
Suggested team member(s) responsible: Health center leadership, community health workers, social work staff.
- Interview patients and families.
- Learn from your community health workers.
- Confirm your existing partnership relationships.
- Develop a stakeholder map and community profile of key current and prospective partners.
- Partner with hospitals, community behavioral health centers, public health, and other key stakeholders to refine your community needs assessments.
3. Convene partners as a workgroup to develop strategies and interventions to address health-related social needs.
Suggested team member(s) responsible: Health center leadership.
- Serve as a convener, bringing partners together for collaboration, and don’t simply rely on individual relationships with external organizations.
- Be clear on your asks and offers to ensure the relationship will be mutually beneficial.
- Develop a shared aim statement with your partners as to why this work is important and your vision of what you hope to achieve.
- Formalize your structure and system of collaboration accountability for progress in the form of ongoing pacing of meetings on a regular basis rather than relying on transactional relationships.
- Develop memoranda of understanding clarifying expectations, roles, and commitments.
- Outline clear next steps and action items with clear roles and responsibilities to maintain accountability across partner organizations.
4. Collaborate with your partners to develop a shared set of strategies on a community approach to address health-related social needs.
Suggested team member(s) responsible: Health center leadership.
- Benchmark and collate approaches to identify potential interventions for addressing health-related social needs.
- Leverage CalAIM resources as part of your intervention plans.
- Develop a driver diagram to guide your efforts and to set priorities.
5. Collaborate with partners to collaboratively design and execute interventions and approaches to address health-related social needs.
Suggested team member(s) responsible: Health center leadership.
- Use workflow mapping tools, swim lane diagrams, and checklists that clarify roles and plan initiatives.
- Develop clear action plans clarifying leaders of intervention strategies, timetables, and measures of progress and success.
Tips
- Health centers are very adept at developing relationships in the community, but may find it challenging to nurture long-term partnerships. A partnership starts with relationships, moves beyond referral for resources or support, and results in co-ownership for addressing community challenges.
- Be clear on being able to have an offer as well as ask when approaching a prospective partner. Avoid transactional relationships with community partners and ensure the partnership has value for all parties.
- Seek to understand your partner’s needs for results, data and reporting. Explore how the partnership can help your partner meet a need or resolve a pain point in their core mission.
- Reach out to your local managed care plan (MCP) to determine processes for payment and care management.
- Learn what is in place for your county through the Medi-Cal program and your managed care plans. If resources are not available, explore technologies that facilitate community referrals such as findhelp.org and Unite Us Cross-sector collaboration software.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
- Going deeper in strengthening partnerships: Addressing community needs requires sustainable and effective partnerships over time. A future activity includes how your organization works with other community partners in an ongoing forum to address community needs (e.g., forming a community partner advisory committee). This often involves the health center as a backbone organization supporting a local collaboration governance structure for shared prioritization and action planning.
- On the horizon in strengthening partnerships: A mature partnership structure in a health center includes assessing the effectiveness of the partnerships and finding ways to continuously ensure alignment so that partnership remains a positive force for all participants. Where possible, making adjustments, such as improved data sharing and pooling of resources to increase leverage in the community, that strengthen the partnerships can be explored.
Resources
KEY ACTIVITY #16:
Continue to Develop Referral Relationships and Pathways
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; pre-visit planning and care gap reduction; care coordination; address social needs.
Overview
Delivery of primary healthcare encompasses not only preventive services and coordination of medical and behavioral healthcare but also referrals for social supports, dental care, follow-up of abnormal findings on screening tests, and other needs of patients, such as weight management or parenting programs. As a result, coordination of care is more complex and often involves referrals outside the practice structure. Examples include referrals from the practice to a provider, such as medical specialty providers, behavioral health providers and social services providers or to a program that will support health and well-being. A referral network also includes acute and subacute facilities, such as hospitals, emergency departments and residential treatment facilities where a patient may receive treatment and the outcome of treatment is necessary for the ongoing care of the patient in the practice.
This activity addresses common steps that your practice can take to optimize both referral pathways to providers and outcomes. The practice of closed loop referrals, in which there is a feedback mechanism to ensure that a referral made by one provider to another is completed and followed up appropriately, leads to greater patient and provider satisfaction. This activity builds on an inventory of existing referral services outside the practice and identifies steps that can be taken to support the systematic management of the referral process.
When a patient requires a referral to obtain needed services outside the practice, the process is a point of vulnerability and a significant percent of specialty referrals aren’t completed, in part due to missing information, misguided referrals and faulty communications. If a referral is not completed, patients may not receive needed services, which can lead to decreased quality of care, decreased patient and provider satisfaction, and ultimately poorer health outcomes. The National Committee for Quality Assurance (NCQA) recognizes the importance of referral networks and their management as a core component of person-centered medical home activities. Building a reliable network of service partners and processes to provide information necessary to inform referrals, as well as having mechanisms to track referral results, is an important part of providing coordinated care that is person-centered.
A major contributor to inequity in health outcomes is unequal access to specialty services and resources to help patients with their condition. The heart of this activity is to empower both the care team and patients through development and maintenance of a robust process to help patients access medical, behavioral health and community resources.
Many factors can impact whether a patient accesses a referral, including language, culture, and lack of understanding of why referral is being requested. Lack of understanding of why a referral is being sought is often a barrier for patients to follow up with a referral, particularly if a patient is not experiencing symptoms.
When appropriate, referrals for social needs and to community health programs can help address barriers including those around transportation, language, and basic needs of food and housing stability, all of which are associated with poorer health outcomes.
Electronic support for providing access to the care team of referral sources is important in the EHR, requiring continuous updating of the database, and for care managers. For care managers and coordinators, externally maintained electronic referral databases are available.
The ability to track referrals (closed loop) remains challenging for most EHRs, and often requires partially manual processes in EHRs and care coordination and population management applications. These manual processes are often facilitated through the construction of a separate registry in which teams can log appointments, track the referral and document completion. This approach requires some process for documenting and updating status in the orders in the EHR.
Population-level reports can identify resource needs and gaps and guide efforts for improving sources of services.
Action steps and roles
1. Identify types of referral partners that your practice needs and gaps in resource availability.
- Some examples to consider: High-volume specialties, such as ophthalmology, podiatry, dermatology, social services providers, and substance use providers.
- In areas where you have gaps in referral providers, telehealth services may be an option. For further information on telehealth services including e-consult, synchronous, asynchronous, and e-visits, contact your managed care plan and/or refer to Telehealth Reimbursement Guide (Pages 14 to 16). Referral processes and tracking are also important to establish for telehealth as well as in-person services.
- Note that there is a shortage of behavioral health clinicians in many parts of California. As you continue to expand your network of off-site behavioral health providers to meet pressing behavioral health needs, also consider behavioral health integration strategies, including expanding your practice’s capacity for providing integrated behavioral health services. Please see PHMI People with Behavioral Health Conditions Guide for more about deepening and expanding integrated care.
- Work with the care teams for your various patient populations to identify gaps in referral resources that could enhance implementation of evidence-based care.
- Use social health screening results that have been disaggregated by REAL and SOGI alongside patient and family feedback to identify unmet needs for which resource relationships have not been established.
2. Assess what tools would be beneficial for the core elements of your referral process.
Examples include (see resources below):
- Referral request form
- Determine what information is required by the provider who is receiving the referral. Information may include the requested service, timing (urgent vs. standard), minimum necessary information, such as patient contact information and applicable demographics, and appropriate clinical and social support questions.
- Referral tracking spreadsheet or software
- Information in a tracking system can include date of referral, appointment status, and whether follow-up is needed. Reach out to your Medi-Cal managed care plans as you are developing referral tracking processes. The MCPs are required to show that Medi-Cal patients are being connected to the services that they are eligible for and referred to; this includes preventive services, specialty services and social services. The MCP may be a good resource for tools, such as those needed for tracking and reporting.
- Referral workflow diagram
- A visual representation or workflow diagram can support staff in the referral process. IHI’s Closing the Loop: A Guide to Safer Ambulatory Referrals in the EHR Era provides a visual nine-step closed loop referral process for specialty referrals, as well as suggestions for improving the process.
3. Assess what tools would be beneficial in enhancing and tracking your referral process.
- Referral guidelines
- For social services, positive responses to screening questions and tools may be the prompt for referral.
- Other types of referrals may be optimized by having documented referral guidelines, such as information needed by specialty provider in order to best answer referral questions.
- Communication templates
- For high volume or frequent referral types, a standardized template for communication between the clinic and referral staff can help ensure that essential information is conveyed efficiently. Examples may include referral letters, progress notes, and post-visit summaries of the plan of care.
- Patient education materials (develop in conjunction with referral network)
- Educational materials and resources for patients that explain the referral process and what to expect can enhance patients’ understanding and support referral completion. In addition, consider training medical assistants, as well as any dedicated care coordination staff, in evidence-based communication techniques, such as teach-back or motivational interviewing to leverage team-based care in supporting the patient to follow through with the referral after the visit.
4. Develop approaches to fill resource gaps.
- Outreach to your managed care plan (MCP) to understand what resources are available. MCPs are required to have online provider directories that include specialists, which can be a resource used by a practice to help find contracted specialists near the patient's home. In addition to comprehensive networks of over a dozen core specialty providers and information on behavioral health and substance use resources, many MCPs are building links to access community-based resources in addition to the 14 Medi-Cal Community Supports through referral platforms that act as a hub to coordinate social needs for patients.
- Identify external providers and community resources that can serve as partners in care and use the established referral process to fill gaps in care they can address.
- Meet and schedule time with high volume providers, including community-based organizations to develop a mutual understanding of goals of referrals and opportunities for the development of a shared process (e.g., referral form).
- The American Academy of Pediatrics offers a sample tool to develop a resource list.
5. Regularly review and update the referral processes and network.
Suggested team member(s) responsible: Leadership.
- As new patient needs are identified, use that opportunity to identify potential resources and expand the referral network. If practices have trouble accessing a contracted specialist for their patient because of either overly long wait times or that they are longer accepting new patients, the MCP is required to approve an out-of-network referral to a more available specialist.
- On a periodic basis, review referral tracking reports to identify referral resources that are used most frequently and use that information to reinforce the relationships and provide feedback on value.
- Review referral tracking reports to identify resources for which loop closure is lacking or from whom required information is not being received on a regular basis. Outreach to these organizations to reinforce expectations or, if necessary, identify a replacement resource for the network.
- For going deeper in tracking quality improvement metrics, practices can develop metrics to assess the effectiveness of the referral network. This might include tracking referral completion rates, patient satisfaction, and time from referral to specialist appointment.
See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
KEY ACTIVITY #17:
Provide Care Management
This key activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; proactive patient outreach and engagement; care coordination; behavioral health integration; address social needs.
Overview
What is care management? How does it differ from care coordination?
Led by a care manager – a licensed clinician or a non-licensed trained individual – care management is an intervention intended to support the highest-need individuals within your practice. The services are more focused, require the development of a person-centered care plan, and are of higher intensity than care coordination services. A patient must consent to participate in care management activities. A care manager works directly with the patient and multidisciplinary care team members to identify, plan and implement person-centered goals and care. The care manager supports the individual in identifying and coordinating resources and referrals, as well as supporting self-management activities to attain optimal wellness.
Care management provides a higher level of support to individuals who have medical, behavioral health and/or social needs that impact their ability to access appropriate levels of care. Individuals may be receiving frequent emergency room (ER) and hospital care that could be avoided with care management activities and/or patients may have significant social barriers (e.g., housing, food, substance use) that prevent them from accessing preventive and wellness care.
As part of care management, the patient’s social needs are directly addressed as part of the assessment and prioritized according to the patient's goals. For example, supporting the patient’s concerns around securing stable housing may be a step to achieving better health.
Addressing social drivers of health helps in reducing health inequities among different populations. Individuals with lower socioeconomic status or limited access to resources can be provided with resources to support these areas.
What type of patients are eligible for care management?
Individuals eligible for care management have high levels of need in one or more of the domains of medical, social or behavioral health. Some examples of populations who may be eligible for care management include:
- Individuals with complex medical Needs (also known as complex care management).
- Example: A patient with a congestive heart failure and advanced kidney disease with a history of frequent hospitalizations for shortness of breath.
- Goal of care management: Support optimal diet and medication management to minimize hospitalization. Address social needs that may impact ability to manage their conditions, such as housing stability, food insecurity.
- Individuals who require intensive support post-hospital discharge (care transitions).
- Example: A patient who had a stroke, resulting in left-sided weakness and mobility issues. They will require additional support including home nursing, physical therapy (PT), occupational therapy (OT), medical equipment, and medication monitoring as they make the transition to the home environment.
- Goal of care management: To coordinate medications, medical equipment, and follow-up care to support optimal recovery and minimize risk of rehospitalization.
- CalAIM: Enhanced Care Management and Community Supports
- In California, Medi-Cal beneficiaries who experience high care management needs may be eligible for a Medi-Cal benefit known as Enhanced Care Management (ECM). ECM can be provided to eligible Medi-Cal beneficiaries at risk of poor outcomes due to high levels of medical, social and behavioral health needs. ECM delivers person-centered, trauma-informed care management services and patients can access additional services known as Community Supports to help achieve their medical and social health goals.
Enhanced Care Management (ECM) and Community Supports[43] are foundational parts of the transformation of Medi-Cal focused on:
- Breaking down the traditional walls of healthcare, extending beyond hospitals and healthcare settings into communities;
- Introducing a better way to coordinate care; and
- Providing high-need patients with in-person care management where they live.
If your practice has a lot of patients who are eligible for ECM based on the descriptions below, you can contact your Medi-Cal managed care plan (MCP). Your MCP can provide you with details on patient eligibility and the requirements needed to provide ECM services to your patients. Some practices also provide services related to Community Supports, depending on their patient population.
ECM is available to specific groups of Medi-Cal patients called “ECM Populations of Focus”:[44]
- Adults, unaccompanied youth and children, and families experiencing homelessness.
- Adults, youth, and children who are at risk for avoidable hospital or emergency department care.
- Adults, youth and children with serious mental health and/or substance use disorder needs.
- Adults living in the community and at risk for long-term care institutionalization.
- Adult nursing facility residents transitioning to the community.
- Children and youth enrolled in California Children’s Services (CCS) or CCS Whole Child Model with additional needs beyond their CCS condition(s).
- Children and youth involved in child welfare (e.g., foster care).
- Adults and youth who are transitioning from incarceration.
- Pregnant and postpartum individuals; birth equity population of focus (starting in 2024).
Medi-Cal patients can also be connected to Community Supports services to help address their health-related social needs, such as access to healthy foods or safe housing, to help with recovery from an illness. If your practice wants to provide Community Supports, contact your Medi-Cal managed care plan for information. Available Community Support Services include:
- Housing transition navigation services.
- Housing deposits.
- Housing tenancy and sustaining services.
- Short-term post-hospitalization housing.
- Recuperative care (medical respite).
- Day habilitation programs.
- Caregiver respite services.
- Personal care and homemaker services.
- Nursing facility transition or diversion to assisted living facilities.
- Community transition services nursing facility transition to a home.
- Environmental accessibility adaptations (home modifications).
- Medically tailored Meals or medically upportive food.
- Sobering centers.
- Asthma remediation.
How does an eligible individual get connected to ECM and Community Supports services?
The Medi-Cal managed care health plans are responsible for identifying their patients who are candidates for Enhanced Care Management and meet eligibility requirements. In addition, practices, providers, and community-based organizations have the ability to refer patients for ECM services. Community-based organizations, such as homeless services providers, are a particularly valuable source of referrals into ECM, as they often have trusted relationships with individuals who may not have sought or received medical care and are therefore not known to the MCP because they have had little or no utilization of healthcare services.
How do we refer a patient who we think would benefit from ECM?
If you have a patient who may be a candidate for ECM or Community Support services, you should contact the Medi-Cal managed care plan to inquire about services available through the patient’s health plan.
Action steps and roles
How can we provide care management services, including ECM services, in our practice?
Community practices are often well situated to provide care management services, as they are the site where patients seek or can be connected to care. Practices may decide that they want to provide care management services, including ECM services. The following resources can help assess your capacity to provide care management services within your structure.
Care management in primary care
This resource from AHRQ is a helpful guide when considering implementing a care management program and addresses the following areas:
- What is care management and why would my practice want it?
- Selecting the right care manager and clarifying the care manager role.
- Training the care manager and practice team.
- Structuring care management.
- Implementation and sustainability issues: Getting care management started and making it continue to work well.
- Resources.
- Data collection, management and analytics.
- Paying for care management.
- Evaluation: How is your care management program working?
Care management for complex populations
A resource on starting a care management program for individuals with multiple medical, behavioral health and social needs is available from the Camden Coalition. This resource addresses:
- Program design: Tools to support the understanding and identification of a complex care population, plan a care model and workflow, and anticipate legal and business needs.
- Program operations: Tools to support the implementation of a complex care model, including triage, outreach, engagement, intake and clinical support.
- Data and process improvement: Tools to support metric identification, data collection and analysis, and process improvement.
- Team and leadership development: Tools to support the recruitment, training and management of a complex care team.
- Community mapping and collaboration: Tools to support multisector and community coordination.
- Communication and growth of success: Tools to support broad communication and program scaling.
Care transitions
This Care Transitions Toolkit from the Indiana Patient Safety Center focuses on processes to support care management for individuals transitioning between healthcare settings.
ECM services
Aurerra Health Group’s Enhanced Care Management Provider Toolkit provides a detailed description of the services that are required to become an ECM provider. In this guide, the requirements of ECM providers are outlined in detail.
FIGURE 23: EXAMPLE OF HOW AN EHR CAN ASSIST WITH CARE MANAGEMENT
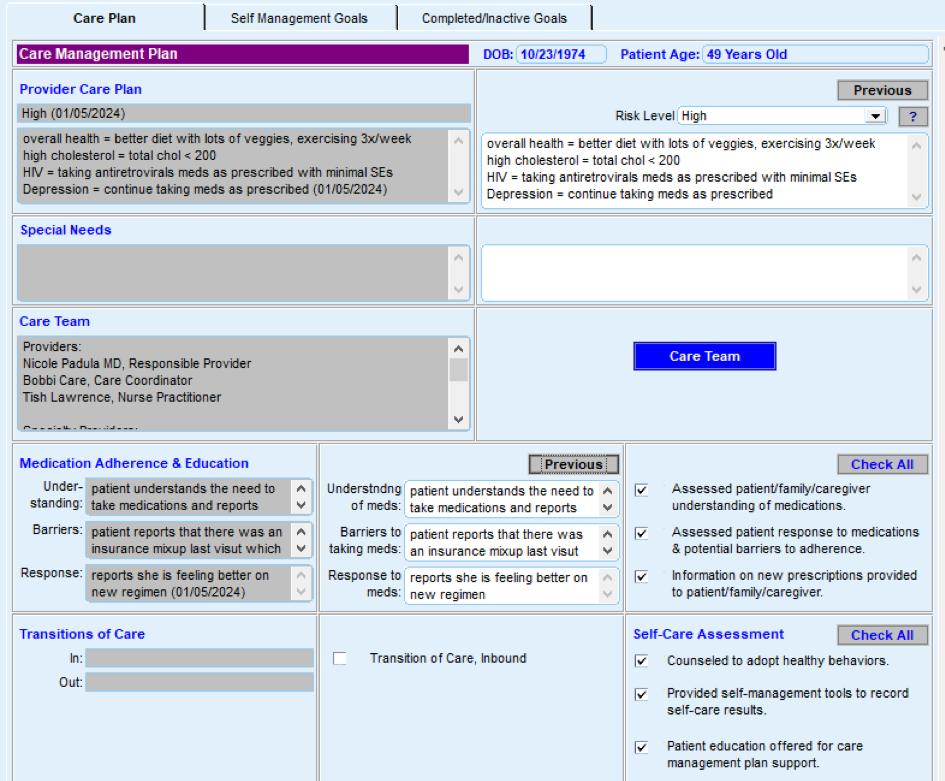
Resources
Evidence base for this activity
KEY ACTIVITY #18:
Strengthen a Culture of Equity
This activity builds off of your practice's approach to address inequities (see Key Activity 4: Use a Systematic Approach to Address Inequities within the Population of Focus) to provide strategies and in-depth resources that can help practices create or strengthen a culture of equity in your practice. The PHMI Equity Framework and Approach emphases that achieving long-term and sustainable improvements to health and racial equity for patients and the broader ecosystem requires a transformational shift at the organizational level. The following strategies can help practices strengthen a culture of equity:
- Demonstrate senior leadership ownership for and commitment to improving health equity.
- Understand and address internalized, personally mediated and institutionalized racism.
- This should include ongoing learning and development activities on the full range of health equity topics (e.g., anti-bias, structural racism, race-based algorithms).
- Build organizational capacity to support efforts to improve health equity.
- Establish practices and policies to promote workforce diversity and provide culturally and linguistically appropriate care.
- Support policy efforts to eliminate inequities driven by social drivers of health.
- This can take the form of joining advocacy efforts or participating in benefits created by recent policy reforms, such as connecting patients with CalAIM Community Supports or Enhanced Care Management. See Key Activity 14: Use Social Needs Screening to Inform Patient Treatment Plans for a discussion on social needs
Resources
On the Horizon Key Activities
Additional activities, including ideas worthy of testing that include the latest ideas and thinking on cancer screening.
KEY ACTIVITY #19:
Explore the Development of Self-Sampling Techniques for Human Papillomavirus (HPV) Screening
This activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; proactive patient outreach and engagement; pre-visit planning and care gap reduction; address social needs.
Overview
This activity offers guidance to practices exploring the development of self-sampling techniques that screen for the presence of human papillomavirus (HPV) as an alternative to a clinician-obtained Pap smear. Studies have demonstrated that self-sampling has the potential to increase participation of underscreened women. In the limited nations where it has been used, self-sampling for HPV has shown high levels of satisfaction among patients.1,6 In the United States, HIV self-testing has been demonstrated to decrease barriers to HIV screening and is supported by the CDC.2 Additionally, self-sampling for detection of chlamydia and gonorrhea has demonstrated both high performance and high patient acceptance. The National Cancer Institute is currently supporting a public-private partnership to bring together federal agencies, industry partners and professional societies to contribute evidence about the accuracy and clinical effectiveness of self-sampling based on HPV testing for cervical cancer screening. This partnership is called the Last Mile Initiative and is designed to evaluate the usability, acceptability and accuracy of multiple self-sampling device-assay combinations and is expected to inform future utility of self-sampling for HPV as an effective tool.
Self-sampling is not yet approved by the FDA in the United States, and it is unclear if or when self-sampling will be approved by the Health Resources and Services Administration (HRSA). However, given World Health Organization (WHO) support for self-sampling techniques and the expansion of technology, this is an option whose development is worth following.
Why this matters
Cervical cancer is a common disease caused by the human papillomavirus (HPV), and the presence of certain HPV subtypes on cervical cancer screening is understood to be a marker that may lead to cancer. Thus, screening for these precancerous cells can be lifesaving. Yet many eligible women do not undergo screening for cervical cancer due to barriers, such as transportation, time and fear of undergoing a pelvic exam. A new method of HPV testing can be performed on vaginal samples collected by the patient through self-sampling techniques. These techniques and materials are under evaluation in the United States and may prove to be effective tools for HPV detection among women at risk. In countries where self-sampling is used, the technique has demonstrated high levels of patient acceptance. A randomized controlled study of over 31,000 individuals enrolled in a U.S. healthcare system demonstrated that direct mail self-sampling increased fulfillment of cervical cancer screening by more than 14% among those who were due or overdue for cervical cancer screening.
Action steps and roles
Because HPV self-sampling techniques are not yet licensed by the FDA or approved by HRSA for use in federally qualified health centers (FQHCs), the technique is not available for health centers at this time. However, practices are encouraged to follow the development of these technologies and continue to evaluate them for possible use in patient populations. In the event self-sampling tools are approved by the FDA and approved for use in health centers, suggested action steps are provided.
To follow are potential steps for developing self-sampling techniques for human papillomavirus (HPV) screening:
- Establish a protocol for self-sampling for HPV that adheres to evidence-based guidance and risk and benefit assessment and adheres to effective sample handling and processing.
- Educate clinicians and support personnel about self-sampling options so that they are able to discuss the options with patients and process materials effectively. See the CDC self-sampling education program under resources for this activity.
- Identify patients who are eligible for self-sampling, inform them of the option, and discuss the risks and benefits of self-sampling for HPV.
- Inform patients about the risks and benefits of self-sampling, discuss the sampling technique, respond to questions, and provide materials and guidance for returning the sample.
- Identify workflows that enable patient education, timely return of the specimen, specimen processing, communication of test results, and appropriate patient follow-up based on test results.
- Send patients reminders about the timing of sampling and sampling technique.
- Process self-samples that are returned to the practice.
This technique is still under scientific study and global use is limited.
Should this method be approved by HRSA, PHMI will provide more detailed guidance for practices.
Resources
Evidence base for this activity
- Di Gennaro G, Licata F, Trovato A, Bianco A. Does self-sampling for human papillomavirus testing have the potential to increase cervical cancer screening? An updated meta-analysis of observational studies and randomized clinical trials. Front Public Health. 2022 Dec 8;10:1003461. doi: 10.3389/fpubh.2022.1003461. PMID: 36568753; PMCID: PMC9773849.
- Centers for Disease Control and Prevention. (2023a, June 1). Self testing. Centers for Disease Control and Prevention. https://www.cdc.gov/stophivtogether/hiv-testing/self-testing.html
- Van Der Pol B, Taylor SN, Mena L, Lebed J, McNeil CJ, Crane L, Ermel A, Sukhija-Cohen A, Gaydos CA. Evaluation of the Performance of a Point-of-Care Test for Chlamydia and Gonorrhea. JAMA Netw Open. 2020 May 1;3(5):e204819. doi: 10.1001/jamanetworkopen.2020.4819. PMID: 32407506; PMCID: PMC7225902.
- NCI Cervical Cancer “last mile” initiative. Division of Cancer Prevention. (n.d.) https://prevention.cancer.gov/major-programs/nci-cervical-cancer-last-mile-initiative
- Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: Focused literature review. Can Fam Physician. 2017 Aug;63(8):597-601. PMID: 28807952; PMCID: PMC5555324.
- Wong EL, Cheung AW, Wong AY, Chan PK. Acceptability and Feasibility of HPV Self-Sampling as an Alternative Primary Cervical Cancer Screening in Under-Screened Population Groups: A Cross-Sectional Study. Int J Environ Res Public Health. 2020 Aug 27;17(17):6245. doi: 10.3390/ijerph17176245. PMID: 32867315; PMCID: PMC7503998.
- Winer RL, Lin J, Anderson ML, et al. Strategies to Increase Cervical Cancer Screening With Mailed Human Papillomavirus Self-Sampling Kits: A Randomized Clinical Trial. JAMA. 2023;330(20):1971–1981. doi:10.1001/jama.2023.21471.
- Serrano B, Ibáñez R, Robles C, Peremiquel-Trillas P, de Sanjosé S, Bruni L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev Med. 2022 Jan;154:106900. doi: 10.1016/j.ypmed.2021.106900. Epub 2021 Nov 30. PMID: 34861338.
KEY ACTIVITY #20:
Develop Customized Culturally Appropriate Educational Materials
This activity involves the following elements of person-centered population-based care: operationalize clinical guidelines; proactive patient; care coordination; address social needs.
Overview
This activity provides guidance on how practices can curate and use existing educational materials and/or develop and use culturally appropriate educational materials to increase the rate of patients who complete recommended colorectal cancer, breast cancer and cervical cancer screening.
Numerous studies[45][46][47][48][49][50][51] have demonstrated that patient education – both self-directed and guided by a member of the care team – is an effective and efficient strategy for getting patients screened for colorectal, breast and/or cervical cancer. Patient education about their risk of cancer, the recommended screening tests, the risks and benefits of the testing methods, and how the tests are performed helps to support informed decision-making, and education is more likely to be followed if it is delivered in ways that are meaningful to the patient, culturally relevant and linguistically appropriate.
Many EHR platforms allow for the development or customization of embedded patient education material. Those tools can then be available for all care team members to share with patients in one of several modalities: at the health center, by mail, or via patient outreach and engagement technologies. Patient outreach and engagement technologies can assist the care team in assessing screening hesitancy, as well as deliver education. These technologies can be programmed to deliver scripted responsive interactions that can extend the ability of the care team to spend time addressing concerns and questions. Care dashboards and quality reports can be utilized with data analytics to identify trends that might suggest population level strategies. Clinical templates and/or prompts in the EHR and care coordination applications can guide provider and care team discussions.
Action steps and roles
1. Use data to determine the populations of focus for new educational materials.
Suggested team member(s) responsible: Panel manager or data analyst and multidisciplinary team for cancer screening.
All patients can benefit from educational materials, and we cover the tailoring and use of existing educational materials in Key Activity 10. Use Culturally Appropriate Educational Materials for Cancer Screening.
This step, however, is focused on determining which populations will require new customized culturally relevant materials to increase their rates of recommended cancer screenings. To determine who might require more new materials, determine which populations or sub-populations within your practice have the lowest rates of screening.
These populations vary by practice but often include:
- Patients of color.
- Patients for whom English in not their primary language.
- Immigrants and refugees.
- Patients with no insurance or high-deductible insurance plans.
- Patients with behavioral health needs.
- Patients experiencing homelessness or housing instability.
- Patients with physical or developmental disabilities.
- Other people who are underserved or medically disadvantaged.
It might also be useful to develop more customized strategies for three populations:
- Patients who are regularly seen by the practice for other health-related reasons (active patients).
- Patients who have been seen by the practice in the past but not over the last 12 months (inactive patients).
- People who are on the practice’s panel but have not been seen by the practice (empaneled, but not yet seen patients).
2. Gather insights on each population of focus identified by the gap reports.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening.
Using one or more human-centered design methods, such as focus groups, 7- Stories, Journeymapping, etc. (see resources on human-centered design below), and work with patients of the population of focus to better understand:
- Their assets, needs and preferences.
- Cultural beliefs, including traditional healing practices.
- Beliefs and level of trust in healthcare generally and in cancer screenings specifically.
- Barriers to getting screened for this population.
- Trusted sources of information or communication mechanisms for this population.
- How they like to receive information (e.g., written, video, as a part of a conversation, etc.).
- Their ideas for increasing cancer screening rates.
3. For each population of focus, use the insights to develop new ideas.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening.
Based upon the insights your practice has developed for a population of focus, use one or more human-centered design methods (see resources on human-centered design below), to develop ideas for new educational materials for them. Creating these ideas is best done with representatives of the population of focus, as they have expertise and experience that may be missing from the practice’s care team.
Before developing the ideas, it is useful to remind the team involved of the common elements and approaches involved in tailoring educational content and messaging for any population of focus. This includes:
Language and tone:
- Developing materials in language(s) spoken by the population of focus.
- Using simple language free from medical jargon. Ensure readability and comprehension of material for all education levels.
- Ensuring that the tone is empathic, respectful and culturally sensitive.
- Using familiar concepts for the population of focus, including incorporating familiar symbols, metaphors and analogies that resonate with their culture(s).
Use of visuals: Use images, familiar symbols, graphics, illustrations, video and/or other visual media that are culturally relevant to population of focus.
Incorporate storytelling:
- Use personal stories from patients of the population of focus who have undergone screenings to emphasize the benefits.
- Share success stories of community members who have undergone screenings to encourage others.
Identify the most appropriate messengers and mechanisms: This includes asking and answering the following questions:
- Who are the best people or groups to get the message across? Should we partner with others for this?
- What communication mechanisms are most appropriate for the population of focus?
- Are there culturally significant events or holidays to convey the message of prevention and care?
- Where should we distribute these materials (e.g., practice waiting areas, community events, local organizations trusted by the population of focus, etc.)?
Accuracy and alignment with clinical guidelines: This involves having new materials reviewed by experts to ensure they adhere to clinical guidelines.
4. Organize the ideas for testing that your practice has come up with.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening.
Now that you have a lot of ideas, it may be helpful to organize them by theme or other criteria. This might include organizing the ideas for new materials in the following categories:
- Materials related to education on the importance of cancer screening.
- Materials related to education on the testing modalities available.
- Materials related to education on the benefits and potential risks of screening.
- Materials related to education on common misperceptions related to cancer screening.
- Materials related to addressing specific barriers to screening, including coverage and potential out-of-pocket expenses.
- Miscellaneous materials or ideas that don’t fit into the above categories.
5. Prioritize ideas for testing.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening.
The steps above have likely generated dozens of ideas for new materials. Your practice likely doesn’t have the bandwidth to test all of them, at least not at the same time,so now is the time to prioritize a few to begin testing. There are many ways to prioritize ideas. The Institute for Healthcare Improvement often recommends a .
If you have organized your ideas for new materials into themes or categories (see step above), you may choose to work on one category or select one or two ideas per category to work on.
The number of ideas that you prioritize for testing first should be based on the team’s bandwidth to engage in testing. So, it is critical to determine the bandwidth for the team(s) who will be doing the testing so that you can determine how many ideas to test first.
6. Begin testing your prioritized ideas for the population of focus.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening.
Whenever testing new educational material or communication mechanisms, we recommend that the practice:
- Start with smaller scale tests (e.g., test with one patient, for one afternoon, in a mailing to 10 patients, etc.).
- Study the successes and challenges of the test. When feasible, this should include getting feedback from patients directly after testing new materials or a new approach with them and incorporating their feedback into your next test.
- Refining the process or material, as needed, based on the test
- Testing again, increasing the scale of the test as these tests result in fewer challenges and better results.
7. Once you have tested, refined and scaled up the initially prioritized ideas, begin testing other ideas.
Suggested team member(s) responsible: Multidisciplinary team for cancer screening.
This might include going back to the ideas developed previously but not prioritized and/or going back through steps three to six above to develop and prioritize new ideas and potentially for additional populations of focus.
8. Put in place formal and informal feedback loops with patients and the care team.
Suggested team member(s) responsible: QI lead or their designee.
To help ensure that new educational materials are meeting the needs of patients and the processes involved are consistently feasible for the care team, it is important to have both formal and informal feedback loops.
For patients, feedback loops might include:
- Sharing draft materials with the population of focus to gather feedback and ensure accuracy before testing.
- Getting feedback from patients directly after testing new materials or a new approach with them and incorporating their feedback into your next test.
- Patient satisfaction surveys (or similar).
- Follow-up calls with a subset of patients to understand what works well and what could be improved.
- Patient focus groups.
- Having the practice’s patient advisory board (or similar) provide feedback.
For the care team, feedback loops might include:
- Existing or new staff satisfaction and feedback mechanisms.
- Regularly scheduled meetings or calls to get staff feedback on processes, methods and tools.
Implementation tips
- If your practice has successfully developed tailored messaging and communication mechanisms for a population of focus on cancer screening or other clinical topics in the past, consider using this as you develop additional tailored materials and mechanisms related to cancer screening.
- Conduct focus groups or other methods to gather information from patients and learn about their cultural beliefs and methods that they prefer.
- When materials are being translated, ask others who have fluency in the language to read the materials and confirm their understanding.
- Borrow from other health centers but be sure to test aspects in your own community. Language, materials, and techniques may resonate better in one community than another.
- See also Appendix D: Peer Examples and Stories from the Field to learn about how others are implementing this activity.
Resources
Appendices
APPENDIX A:
Sample Idealized System Diagram
WEAVING YOUR MEASUREMENT STRATEGY AND LEARNING SYSTEM INTO PRACTICE OPERATIONS
The below graphic provides an example to visualize the flow of information between a practice’s measurement strategy and actions at the practice and care team levels, including how to use the information for continual learning and improvement.
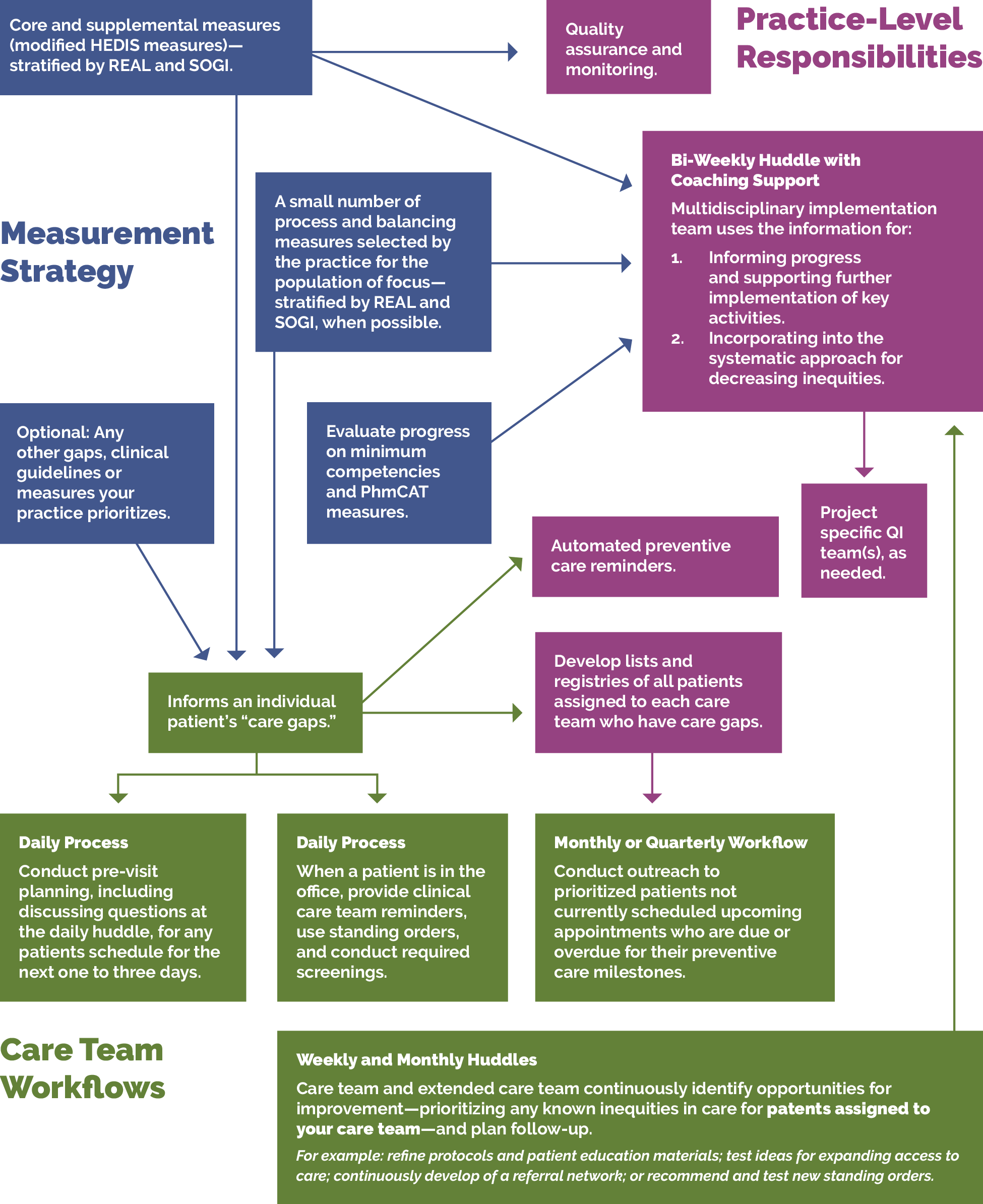
APPENDIX B:
Theory of Change
The key activities in this guide align with the PHMI theory of change for how practices can increase colorectal cancer, breast cancer, and cervical cancer screening rates through three primary drivers:
- Effective implementation of well-visit protocols.
- Patients eligible and due for visits and immunizations have the information, education and access needed for screening.
- Practices have needed resources and support.
FIGURE 24: PHMI ADULT PREVENTION DRIVER DIAGRAM
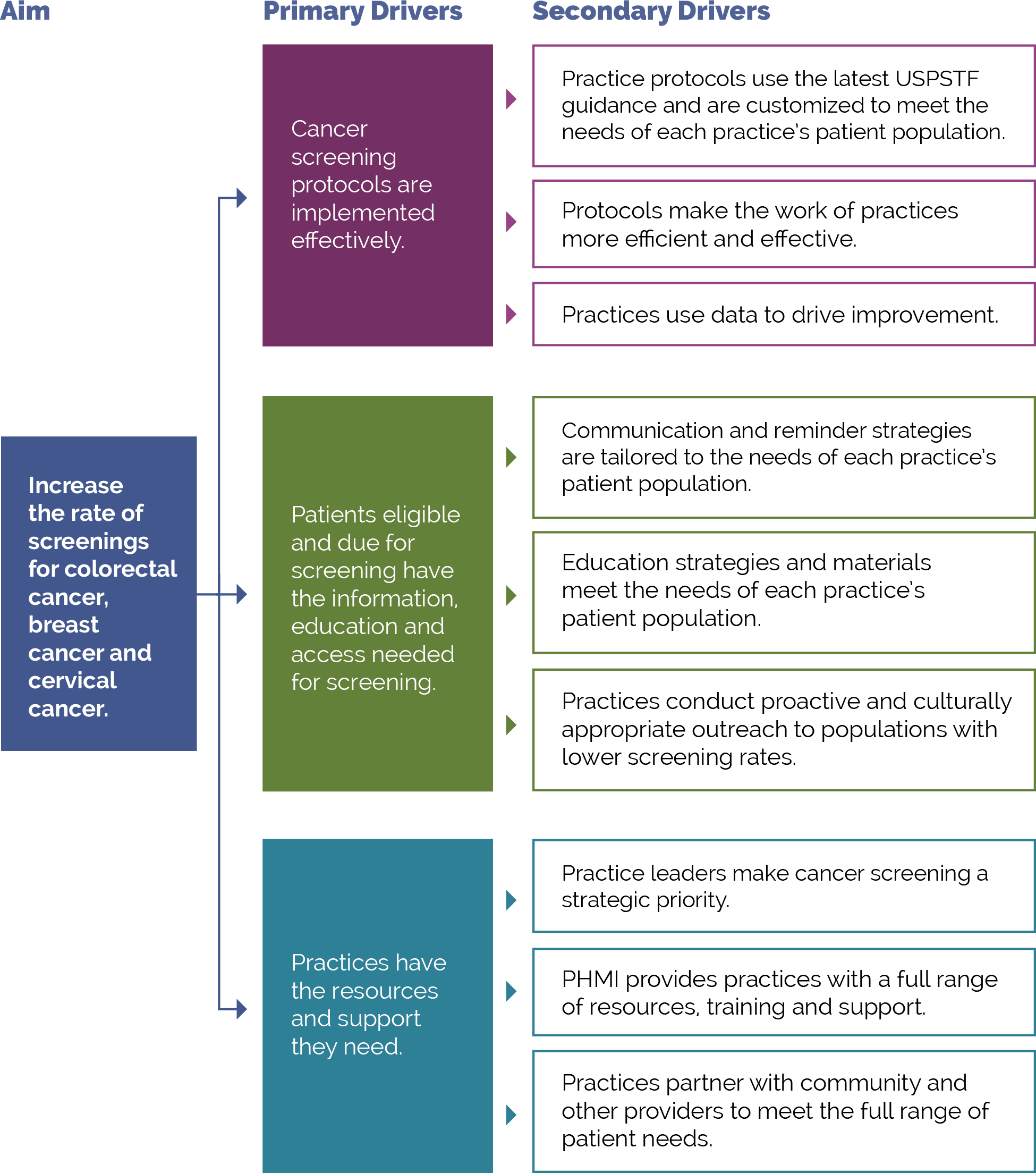
APPENDIX C:
Developing a Robust Measurement Strategy
FIGURE 25: DEVELOPING YOUR MEASUREMENT STRATEGY MILESTONES
Figure 25 illustrates the key milestones in the development of a robust measurement strategy.
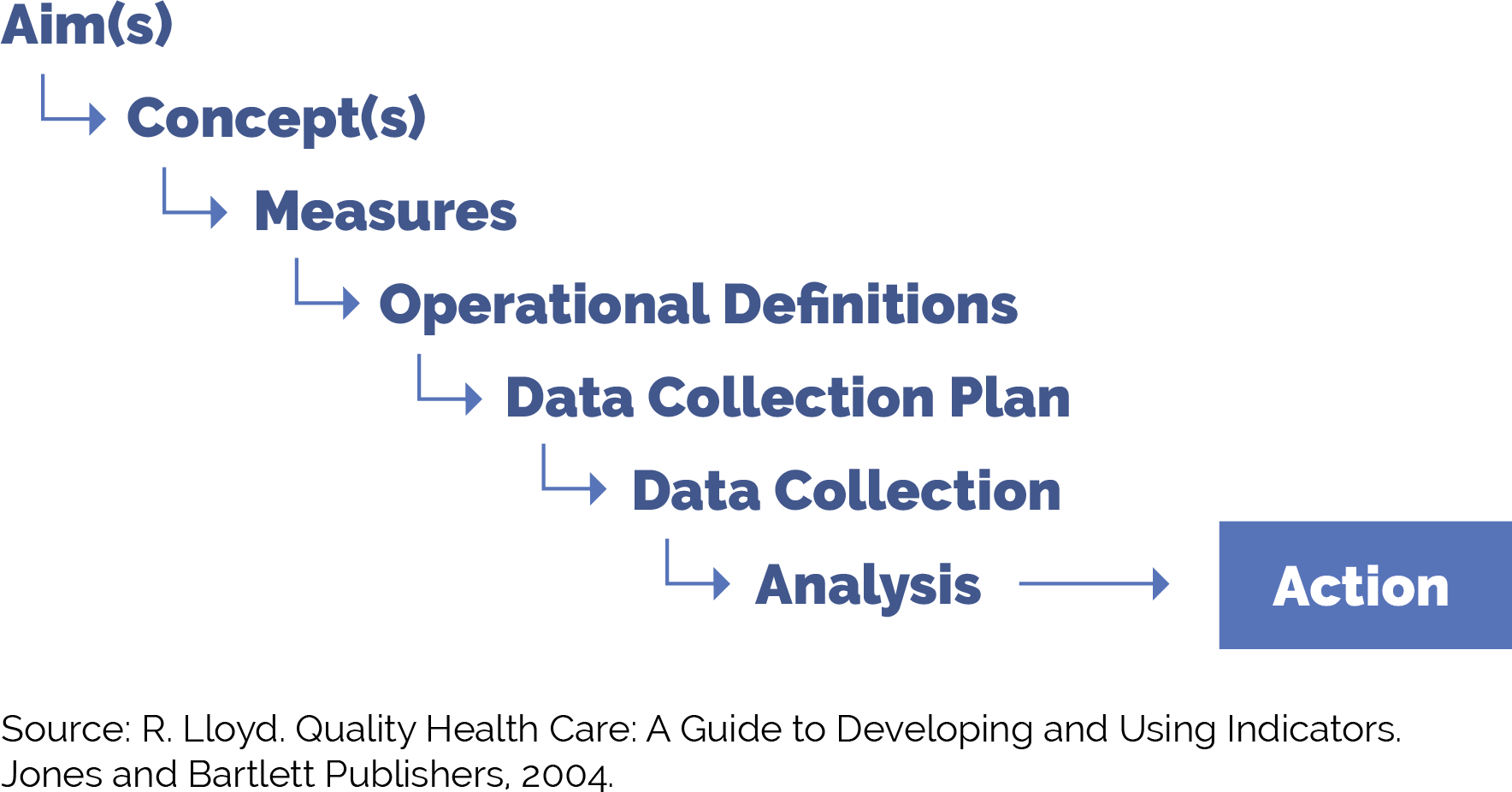
FIGURE 26: DEFINITION AND EXAMPLES FOR MEASUREMENT STRATEGY MILESTONES
Figure 26 provides guidance on each of these milestones as you work to put in place a robust yet practical measurement strategy to improve outcomes for adolescents and adults with behavioral health needs.
Milestone |
Definition and Examples |
Example for Adults with Preventive Care Needs |
|---|---|---|
Aim(s) |
The overall goal(s) of the improvement effort. “What are we trying to accomplish?” We often recommend sub-aims to focus your team on intermediate goals. You can develop data-informed specific, measurable, achievable, relevant, time-bound, inclusive and equitable (SMARTIE) goals focused on increasing adherence to specific AAP preventive care guidelines year over year among attributed patients or subpopulations of patients. |
Identifying and addressing colorectal cancer screening for all patients who are due to increase the percentage of those screened from x% to y% by [insert date]. Example sub-aim: Increase the percentage of our Black patients who are due for colorectal cancer screening from 33% to 70% by December 2025. |
Concept(s) |
A general abstract notion (approach, thought, belief or perception) related to the aim(s) of focus. |
Access to CRC screening options; patient attitudes about CRC screening; patient reminders; speed and efficiency of your workflows. |
Measures |
Specific objective ways to determine the extent to which an aim has been met or to determine if there has been improvement in the concepts of focus. Measures help us to answer the second question in the model for improvement: “How will we know that a change is an improvement?” Measures generally fall into one of three types:
|
See below for example outcomes, process and balancing measures. For the following examples, we will examine a measure tied to patient satisfaction: wait time.
|
Operational definitions |
A detailed description, in quantifiable terms, of what to measure and the steps to follow to measure it consistently each time and over time. The operational definitions help make the measure clear and unambiguous and often contain criteria for inclusion and exclusion and numerator/denominator. |
Patient wait time operational definition: Refers to the total amount of time that a patient spends from the moment they arrive at the health center until they are seen by a healthcare provider or receive the intended healthcare service. The practice will track wait time for all patients with scheduled appointments. |
Data collection plan |
A detailed set of instructions that generally includes:
|
Patient wait time data collection plan: Who (and how):
Where and how the data will be stored:
When the data will be collected:
How often:
|
Data collection |
The process of collecting the agreed upon measures in accordance with the relevant operational definitions and the agreed upon data collection plan. |
Patient wait time data collection: Wait time data is collected semiautomatically when the receptionist notes on the EHR when the patient signs in and when the care team member opens the visit note. |
Analysis and action |
The process of analyzing the data, including instructions for the analysis and visualization of the data, disseminating the data to relevant parties, and using the data to track progress and guide improvement efforts. |
Patient wait time analysis and action: The process where the improvement team reviews data on wait times on a weekly basis and the care team and panel manager review the data subsequently once per quarter or every six months for ongoing monitoring. |
POPULATION HEALTH GUIDE FOR UNDERTAKING 3 PART DATA REVIEW
The three part data review is a tool that can be used to advance work related to population health. It draws on asset-based inquiry, to surface to identify needs and opportunities to understand the ways in which systems have discarded or undervalued the assets of individuals and communities. Groups can then work together to ensure that all can contribute to advancing population health and well-being and dismantling inequities. Access the full resource.
APPENDIX D:
Peer Examples and Stories from the Field
Practices across California and the U.S. offer a treasure trove of examples of how to improve colorectal cancer, breast cancer and cervical cancer screening rates. In many cases, these examples show how practices implemented multiple activities in this guide to increase cancer screening rates.
The table below provides links to some of these examples, along with the activities in this guide that the example is related to or touches on.
FIGURE 27: CORE POPULATION HEALTH MANAGEMENT FUNCTIONALITY REQUIREMENTS
Example |
Activities in This Guide the Example Relates To |
|---|---|
|
How The Los Angeles LGBT Center Championed Population Health to Maximize Colorectal Cancer Screening. |
|
|
North Coast Clinics Network shares how health centers can provide the necessary care for cervical cancer screening in rural northern California. |
|
|
North Coast Clinics Network shares how health centers can provide the necessary care for breast cancer screening in rural northern California. |
|
|
North Coast Clinics Network shares how health centers can provide the necessary care for colorectal cancer screening in rural northern California. |
|
|
Shasta Community Health Center describes how they used scribes to improve cervical cancer screening. |
|
|
OLE Health shares how they improved cervical cancer screening rates. |
|
|
OLE Health shares how they used care coordinators to increase colorectal cancer screening rates. |
|
|
San Mateo Medical Centers: Using 22otters for Colonoscopy Prep. |
|
|
Santa Rosa Community Health’s increased its colorectal cancer screening rates by developing a new way of packaging FIT Tests. |
|
|
Community Medical Centers (CMC) implemented a “Do the Poo” initiative to increase colorectal cancer screening rates. |
|
APPENDIX E:
Guidance on Technological Interventions
EHRs were primarily designed to manage individual patients rather than groups of patients. However, over time EHRs have increasingly added functionality for population-level quality reporting and management and some degree of care planning and care coordination, especially to support value-based care tracking and reporting. Practices should evaluate your EHR capabilities against specifically designed population management applications. While these applications require interface with the EHR, they generally offer additional functionality. While EHR solutions are integrated with EHR data, they still usually require import of data from outside sources to be optimally useful. Managed care organizations may provide care coordination and population management applications, but usually only for their own enrolled patients. EHR-based solutions may also pose challenges where groups of practices using different EHR solutions are collaborating in value-based care contracts.
In value-based care arrangements, practices are responsible for attributed patients who may have never been seen. Since these patients do not have records in the EHR, practices need to consider how they can manage these patients to engage them into care at the practice in the absence – at least initially – of the patients having records within the EHR. If your practice is using freestanding applications for this, they need the capacity to handle these attributed patients who have not been registered as patients.
FIGURE 28: CORE POPULATION HEALTH MANAGEMENT FUNCTIONALITY REQUIREMENTS
Figure 28 below includes the technical functionalities required to support population management for adults with preventive care needs. These requirements can guide the evaluation of existing solutions or guide the development of requirements in evaluating potential new applications. The table also indicates the data sources required to enable the functionality.
Functionality |
Population Health Management Requirement Description |
Data Acquisition Dependency |
|---|---|---|
Care guidelines |
Identify care gaps for all adults with preventive care needs against care protocol. Care guidelines may be presentable to the clinical provider or support team at point of care through the EHR, in visit workflow as pre-visit prep or team huddle, through registries as above, and as aspirational prompts to patients or caregivers. |
Commercial EHR-embedded guidelines provided by vendor or customized by practice. External source guidelines (clinical guidelines). Reference sites made available electronically. |
Registries |
Ability to produce registries (a list or cohort of patients) organized to facilitate population management. For example: adults in age ranges relevant to the measures and/or clinical standards or adults sharing designated high-risk criteria (e.g., medical, behavioral, social needs) impacting their ability to achieve guidelines. These registries should consider the inclusion of functionality to trigger automated, pre-defined action(s) and/or human-initiated action(s) for all or a defined subset of patients comprising the registry. Suggested HIT assets that can be leveraged to achieve this function include:
|
EHR:
External data sources, such as: reference labs, specialty care, immunization registries, or social service providers’ data |
Clinical decision support (CDS) |
Care gaps should be displayed based on what is due with insight into previous results to support clinicians’ ability to make decisions at the point of care (POC) for the provider and care team members supporting non-POC management. Care guidelines may be presentable as clinical decision support to the clinical provider and support team at point of care, in visit workflow as pre-visit prep or team huddle, through registries as above, and as aspirational prompts to patients and caregivers. While EHR-based prompts are usually thought of as ideal, team-based care presents an opportunity for CDS to be presented to other members of the care team through other channels. The Five Rights Framework Clinical Decision Support: More Than Just ‘Alerts’ Tipsheet is a useful guidance to help health centers to support decision-making across a wider range of the care delivery lifecycle, broader teams and technology other than the EHR to look beyond office visits and providers. This is especially important to avoid alert fatigue and burnout. |
Internal EHR data. External source clinical data. Claims data (clinical lag should be noted). Electronic guideline specifications. Patient-contributed data. |
Care dashboards and reports |
Adult preventive care dashboard: Population view by eligible study with sorting and filtering capability based on characteristics to be defined by the practice, with the ability for the care team and case managers to document the actions completed. Ability to see care gaps at a patient level and population level according to health center-prioritized care guidelines. Note that, to automate these reports, it is necessary to apply standardized data collection strategies against electronically specified protocols. |
Same as above (EHR data and external data sources. Data from other sources of care). Claims data. |
Quality reports |
Same as above by quality measures, as opposed to care guidelines; ability to track HEDIS as well as customized measures and UDS. |
Quality measure specifications. Same as above (EHR data and external data sources). Data from other sources of care. Claims data. |
Risk stratification |
Ability to categorize risk for patients and develop lists according to risk classification (tie to registry). Can be imported as externally generated risk score or calculated internally according to proprietary or customized risk algorithm. |
Data acquisition platform ingestion: already curated high-risk list ingested and utilized downstream in the journey and/or additional internal and external data sources to populate defined risk model. |
Outreach and engagement |
Allow for outreach to support previsit planning or post-visit care needs, such as assessments. Technology channels include population registry outputs; patient-facing applications, such as patient portals; freestanding text messaging; and self-assessment/self-management applications.
|
Same as above (clinical/EHR/etc.) Claims.
|
Care management |
Allow for management of specific and unique care needs for high-risk patients. Care management requires the ability for multiple members of the care team to contribute to and rack elements of the plan. Challenges with freestanding care management applications include access to data from other sources of care, including the ability to track referrals, and workflow burden of staff utilizing multiple applications. Ability of the care management application to draw from and “write back” to the EHR is desirable but difficult to achieve.
|
Care management protocols. Appointment data: internal/external. Clinical data from external service providers.
|
FIGURE 29: USE OF TECHNOLOGY FOR RECOMMENDED SCREENING FOR KEY ACTIVITIES
This table identifies strategies for using digital tools to complete appropriate screeners as recommended by clinical guidelines. Using technology to facilitate screening may streamline the workflow and preserve patient confidentiality where necessary.
ID |
Focus Area |
Completion of Digital Screeners |
Data Acquisition Dependency |
|---|---|---|---|
1 |
Depression screening |
In-office tablet-based screening and/or remote patient-facing application-based self-completed screening.* |
Population health and EHR integration of screener responses or, at minimum, scores. |
2 |
Anxiety screening |
In-office tablet-based screening and/or remote patient-facing application-based self-completed screening.* |
Population health and EHR integration of screener responses or, at minimum, scores. |
3 |
Unhealthy substance user screening |
In-office tablet-based screening and/or remote patient-facing application-based self-completed screening.* |
Population health and EHR integration of screener responses or, at minimum, scores. |
4 |
Social needs screening |
In-office tablet-based screening and/or remote patient-facing application-based self-completed screening.* |
Population health and EHR integration of screener responses or, at minimum, scores. |
*A workflow should be codified for identifying emergent behavioral health risks. A workflow should be codified for preserving patient confidentiality.
FIGURE 30: USE OF TECHNOLOGY FOR PATIENT OUTREACH AND PRE-VISIT PLANNING FOR FOUNDATIONAL KEY ACTIVITIES
This table outlines the use of technology to facilitate specific activities and potential technology solutions that can optimize the uptake and efficiency of in-office visits.
ID |
Technology Focus |
Patient Inreach and Pre-Visit Planning |
Data Acquisition Dependency |
|---|---|---|---|
1 |
Portal-based communication |
|
EHR interface and integration. |
2 |
AI-enabled chatbots |
Identifying issues that need to be addressed before an office visit that can be converted to telehealth visits. |
Population health and EHR incorporation of screening scores and responses. |
3 |
Text messaging |
Appointment reminders. |
EHR interface and integration. |
FIGURE 31: USE OF TECHNOLOGY FOR ENHANCED PATIENT ENGAGEMENT AND VIRTUAL CARE FOR GOING DEEPER ACTIVITIES
The table identifies technology solutions to engage patients asynchronously from office visits for a variety of use cases to enhance care and patient experience.
ID |
Focus Area |
Patient Engagement and Mobile Technology |
Data Acquisition Dependency |
|---|---|---|---|
1 |
AI-enabled Chatbots |
|
HR interface and integration. |
2 |
Remote medical devices |
|
EHR and population health integration. |
FIGURE 32: USE OF TECHNOLOGY FOR INNOVATIONS IN CARE DELIVERY FOR ON THE HORIZON ACTIVITIES
The table describes technology strategies that can enhance care delivery by using artificial intelligence and advanced technology tools.
ID |
Focus Area |
Artificial Intelligence (AI) and Innovation |
Data Acquisition Dependency |
|---|---|---|---|
1 |
Predictive analytics |
|
EHR integration, population health, and patient engagement application integration. |
2 |
Artificial intelligence-enabled diagnostics |
Advanced diagnostic tools that can use imaging, audio files, and EHR data to suggest diagnoses and care management plans. |
EHR integration and population health integration |
RECOMMENDED CITATION AND ACKNOWLEDGEMENTS
Recommended citation: Franckhauser M, Howard P, Baker L, Deane M, Burgess K, Donald F, Rachman F, Esmond W. Adults with Preventive Care Needs Implementation Guide. In: Coleman K, Mital M, editors. Population Health Management Initiative Populations of Focus Implementation Guide Series. 1st ed. Oakland, CA: Kaiser Permanente; 2024.
Acknowledgments: We gratefully acknowledge the contributions of the many staff and representatives of the Institute for Healthcare Improvement (IHI), AllianceChicago, Center for Care Innovations (CCI), JSI Inc and the Center for Excellence in Primary Care at University of California, San Francisco along with Pyramid Communications and Kaiser Permanente’s Population Health Management Initiative (PHMI) team for crafting this work.
In addition, we are grateful for the partnership of the Department of Health Care Services (DHCS), Community Health Center partners in PHMI, representatives from the California Primary Care Association and the Regional Associations of California (RAC) along with the many other academic, health plan and consulting partners who shared their expertise and insights freely as we collaboratively designed the initiative and created these guides.
Special thanks to the individual reviewers of this first edition of the Populations of Focus Implementation Guide Series who significantly strengthened this work, including: Jeff Norris, David Tian, Karen Mark, Palav Babaria (DHCS); Jennifer Sayles (Pop Health Learning Center); Roger Chaufournier, Christine St. Andre (IHI); Kathleen Figoni, Angela Sherwin (CCI).
Endnotes
- USCS Data Visualizations [Internet]. gis.cdc.gov. 2023. Available from: https://gis.cdc.gov/Cancer/USCS/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcancer%2Fdataviz%2Findex.htm#/AtAGlance/
- American Cancer Society. American Cancer Society | Cancer Facts & Statistics [Internet]. American Cancer Society | Cancer Facts & Statistics. 2023. Available from: https://cancerstatisticscenter.cancer.org/#
- Institute of Medicine. 2003. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press. https://doi.org/10.17226/10260
- Improving Health Equity: Build Infrastructure to Support Health Equity. Guidance for Health Care Organizations. Boston, Massachusetts: Institute for Healthcare Improvement; 2019. (Available at www.ihi.org)
- Chadha N, Lim B, Kane M, Rowland B. Institute for Healing and Justice in Medicine; 2020. https://belonging.berkeley.edu/race-medicine
- REaL Data Collection Toolbox [Internet]. KHA; 2022 [cited 2023 Dec 18]. Available from: http://www.khaquality.com/Portals/[4]/Resources/HQIC_REaL%20Data_Collection_Toolbox.pdf
- Leubner J, Wild S. Developing Standing Orders to Help Your Team Work to the Highest Level. Fam Pract Manag. 2018 May/Jun;25(3):13-16. PMID: 29989776.
- https://cepc.ucsf.edu/standing-orders
- Adams SA, Rohweder CL, Leeman J, Friedman DB, Gizlice Z, Vanderpool RC, et al. Use of Evidence-Based Interventions and Implementation Strategies to Increase Colorectal Cancer Screening in Federally Qualified Health Centers. Journal of Community Health. 2018 May 16;43(6):1044–52.
- Power E, Miles A, von Wagner C, Robb K, Wardle J. Uptake of colorectal cancer screening: system, provider and individual factors and strategies to improve participation. Future Oncology. 2009 Nov;5(9):1371–88.
- Subramanian S. Adherence with colorectal cancer screening guidelines: a review. Preventive Medicine. 2004 May;38(5):536–50.
- Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and Physician Reminders to Promote Colorectal Cancer Screening. Archives of Internal Medicine. 2009 Feb 23;169(4):364.
- Time physicians spent with patient U.S. 2018 | Statista [Internet]. Statista. Statista; 2018. Available from: https://www.statista.com/statistics/250219/us-physicians-opinion-about-their-compensation/
- Alberti LR, Garcia DPC, Coelho DL, Lima DCAD, Petroianu A. How to improve colon cancer screening rates. World Journal of Gastrointestinal Oncology [Internet]. 2015;7(12):484. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4678395/
- Baron RC, Melillo S, Rimer BK, Coates RJ, Kerner J, Habarta N, et al. Intervention to Increase Recommendation and Delivery of Screening for Breast, Cervical, and Colorectal Cancers by Healthcare Providers. American Journal of Preventive Medicine. 2010 Jan;38(1):110–7.
- Peterson EB, Ostroff JS, DuHamel KN, D'Agostino TA, Hernandez M, Canzona MR, Bylund CL. Impact of provider-patient communication on cancer screening adherence: A systematic review. Prev Med. 2016 Dec;93:96-105.
- Cooper CP, Gelb CA. Opportunities to Expand Colorectal Cancer Screening Participation. J Womens Health (Larchmt). 2016 Oct;25(10):990-995. doi: 10.1089/jwh.2016.6049. PMID: 27749190; PMCID: PMC6066278.
- Gwede CK, Sutton SK, Chavarria EA, Gutierrez L, Abdulla R, Christy SM, Lopez D, Sanchez J, Meade CD. A culturally and linguistically salient pilot intervention to promote colorectal cancer screening among Latinos receiving care in a Federally Qualified Health Center. Health Educ Res. 2019 Jun 1;34(3):310-320.
- Dalton AF, Golin CE, Morris C, et al. Effect of a Patient Decision Aid on Preferences for Colorectal Cancer Screening Among Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2022;5(12):e2244982. doi:10.1001/jamanetworkopen.2022.44982.
- Reuland DS, Brenner AT, Hoffman R, McWilliams A, Rhyne RL, Getrich C, et al. Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population. JAMA Internal Medicine. 2017 Jul 1;177(7):967.
- Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2010 Nov 8;117(8):1745–54.
- Tong EK, Nguyen TT, Lo P, Stewart SL, Gildengorin GL, Tsoh JY, et al. Lay health educators increase colorectal cancer screening among Hmong Americans: A cluster randomized controlled trial. Cancer. 2016 Aug 26;123(1):98–106.
- Nguyen TT, Tsoh JY, Woo K, Stewart SL, Le GM, Burke A, et al. Colorectal Cancer Screening and Chinese Americans: Efficacy of Lay Health Worker Outreach and Print Materials. American Journal of Preventive Medicine. 2017 Mar;52(3):e67–76.
- Doubeni CA, Gabler NB, Wheeler CM, McCarthy AM, Castle PE, Halm EA, Schnall MD, Skinner CS, Tosteson ANA, Weaver DL, Vachani A, Mehta SJ, Rendle KA, Fedewa SA, Corley DA, Armstrong K. Timely follow-up of positive cancer screening results: A systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018 May;68(3):199-216.
- Sansoni M, Tagai EK, Lapitan E, Wen KY, Xu J, Belfiglio A, Hudson SV, Kohler RE, Hernandez E, Miller SM. Development of a text message-based intervention for follow-up colposcopy among predominately underserved Black and Hispanic/Latinx women. Cancer Causes Control. 2022 Jun;33(6):861-873. doi: 10.1007/s10552-022-01573-y. Epub 2022 Mar 25. PMID: 35334016; PMCID: PMC9516784.
- National Cancer Institute. HPV and Pap Test Results: Next Steps after an Abnormal Cervical Cancer Screening Test - NCI [Internet]. www.cancer.gov. 2022. Available from: https://www.cancer.gov/types/cervical/screening/abnormal-hpv-pap-test-results
- Patel, M.P., Schettini, P., O’Leary, C.P. et al. Closing the Referral Loop: an Analysis of Primary Care Referrals to Specialists in a Large Health System. J GEN INTERN MED 33, 715–721 (2018). https://doi.org/10.1007/s11606-018-4392-z
- American College of Physicians. Transforming Clinical Practice Initiative SAN Power Packs: Closing the Loop. Centers for Medicare & Medicaid Services; 2019. Available from: https://www.cms.gov/priorities/innovation/files/x/tcpi-san-pp-loop.pdf
- Reducing Care Fragmentation: A Toolkit for Coordinating Care [Internet]. The Center for Accelerating Care Transformation. The Commonwealth Fund; 2010. Available from: https://www.act-center.org/application/files/7016/3112/2157/Toolkit_Reducing_Care_Fragmentation.pdf
- Alegría, M., NeMoyer, A., Falgàs Bagué, I., Wang, Y., & Alvarez, K. (2018). Social Determinants of Mental Health: Where We Are and Where We Need to Go. Current psychiatry reports, 20(11), 95. https://doi.org/10.1007/s11920-018-0969-9
- Ruiz Escobar E, Pathak S, Blanchard CM. Screening and Referral Care Delivery Services and Unmet Health-Related Social Needs: A Systematic Review. Preventing Chronic Disease. 2021 Aug 12;18.
- Chavez, L. J., Tyson, D. P., Davenport, M. A., Kelleher, K. J., & Chisolm, D. J. (2023). Social Needs as a Risk Factor for Positive Postpartum Depression Screens in Pediatric Primary Care. Academic pediatrics, S1876-2859(23)00095-5. Advance online publication. https://doi.org/10.1016/j.acap.2023.03.007
- Ruiz Escobar E, Pathak S, Blanchard CM. Screening and Referral Care Delivery Services and Unmet Health-Related Social Needs: A Systematic Review. Preventing Chronic Disease. 2021 Aug 12;18.
- Califf, R. M., Wong, C., Doraiswamy, P. M., Hong, D. S., Miller, D. P., Mega, J. L., & Baseline Study Group (2022). Importance of Social Determinants in Screening for Depression. Journal of general internal medicine, 37(11), 2736–2743. https://doi.org/10.1007/s11606-021-06957-5
- Ponce-Chazarri L, Ponce-Blandón JA, Immordino P, Giordano A, Morales F. Barriers to Breast Cancer-Screening Adherence in Vulnerable Populations. Cancers. 2023 Jan 18;15(3):604.
- National Academies of Sciences, Engineering, and Medicine; National Academy of Medicine; Committee on the Future of Nursing 2020–2030; Flaubert JL, Le Menestrel S, Williams DR, et al., editors. The Future of Nursing 2020-2030: Charting a Path to Achieve Health Equity. Washington (DC): National Academies Press (US); 2021 May 11. 2, Social Determinants of Health and Health Equity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK573923/
- Butler, AB ED, Morgan, MD, MSc, MSHP AU, Kangovi, MD, MS S. Screening for Unmet Social Needs: Patient Engagement or Alienation? NEJM Catalyst [Internet]. 2020 Jul 20; Available from: https://catalyst.nejm.org/doi/full/10.1056/CAT.19.1037
- Kaiser Permanente Research Affiliates Evidence-based Practice Center. Screening and Interventions for Social Risk Factors: A Technical Brief to Support the U.S. Preventive Services Task Force [Internet]. 2021. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/inline-files/Social%20Risk%20Factors%20Tech%20Brief_Assembled%20for%20Web_Sep%202021_1.pdf
- Rojas L, Project S. Accountable Health Communities (AHC) Model Evaluation Second Evaluation Report RTI Point of Contact [Internet]. 2023 [cited 2024 Jan 16]. Available from: https://www.cms.gov/priorities/innovation/data-and-reports/2023/ahc-second-eval-rpt
- Sınger A, Coleman K, Mahmud A, Holden E, Stefanik-Guizlo K. Assessing the Feasibility of an Empathic Inquiry Approach to Social Needs Screening in 10 Federally Qualified Health Centers. The Permanente Journal. 2023 Dec 15;27(4):136–42.
- Byhoff E, Gottlieb LM. When There Is Value in Asking: An Argument for Social Risk Screening in Clinical Practice. Ann Intern Med. 2022;175(8):1181-1182. doi:10.7326/M22-0147
- American College of Physicians . Transforming Clinical Practice Initiative SAN Power Packs: Closing the Loop [Internet]. Centers for Medicare & Medicaid Services; 2019. Available from: https://www.cms.gov/priorities/innovation/files/x/tcpi-san-pp-loop.pdf
- Ca.gov. 2023. Available from: https://www.dhcs.ca.gov/CalAIM/ECM/Pages/Home.aspx
- California Advancing and Innovating Medi-Cal (CalAIM) [Internet]. Available from: https://www.dhcs.ca.gov/CalAIM/Documents/CalAIM-ECM-a11y.pdf
- Cooper CP, Gelb CA. Opportunities to Expand Colorectal Cancer Screening Participation. J Womens Health (Larchmt). 2016 Oct;25(10):990-995. doi: 10.1089/jwh.2016.6049. PMID: 27749190; PMCID: PMC6066278.
- Gwede CK, Sutton SK, Chavarria EA, Gutierrez L, Abdulla R, Christy SM, Lopez D, Sanchez J, Meade CD. A culturally and linguistically salient pilot intervention to promote colorectal cancer screening among Latinos receiving care in a Federally Qualified Health Center. Health Educ Res. 2019 Jun 1;34(3):310-320.
- Dalton AF, Golin CE, Morris C, et al. Effect of a Patient Decision Aid on Preferences for Colorectal Cancer Screening Among Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2022;5(12):e2244982. doi:10.1001/jamanetworkopen.2022.44982.
- Reuland DS, Brenner AT, Hoffman R, McWilliams A, Rhyne RL, Getrich C, et al. Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population. JAMA Internal Medicine. 2017 Jul 1;177(7):967.
- Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2010 Nov 8;117(8):1745–54.
- Tong EK, Nguyen TT, Lo P, Stewart SL, Gildengorin GL, Tsoh JY, et al. Lay health educators increase colorectal cancer screening among Hmong Americans: A cluster randomized controlled trial. Cancer. 2016 Aug 26;123(1):98–106.
- Nguyen TT, Tsoh JY, Woo K, Stewart SL, Le GM, Burke A, et al. Colorectal Cancer Screening and Chinese Americans: Efficacy of Lay Health Worker Outreach and Print Materials. American Journal of Preventive Medicine. 2017 Mar;52(3):e67–76.